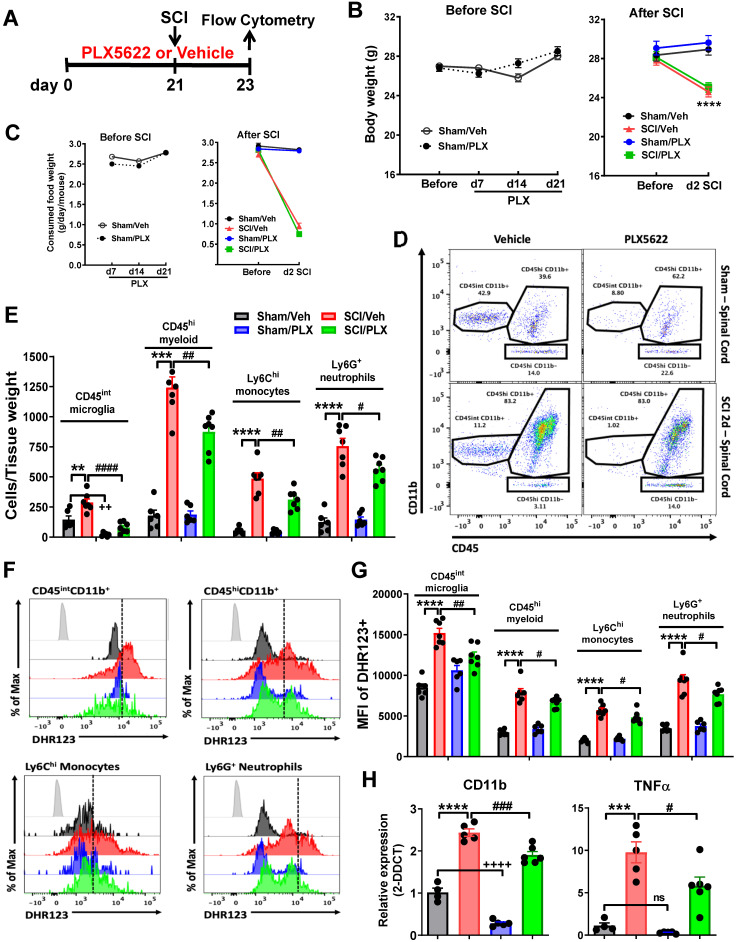
Figure 1.
Pre-depletion of microglia with PLX5622 reduces infiltrating cells and reactive oxygen species (ROS) production after SCI. (A) PLX5622 diet or vehicle chow were fed to animals three weeks before injury and remained for 2 days after injury. (B) Body weight was recorded both prior and after injury. All injured mice appeared significant loss of body weight. PLX had no effects on animal weight both prior and after injury. n = 30 mice/group before SCI and 12 (Sham/Veh), 12 (Sham/PLX), 18 (SCI/Veh), and 18 (SCI/PLX) mice after SCI. (C) Food intake was recorded both prior and after injury. In all injured mice, food consumption decreased significantly. PLX had no effects on food consumption both prior and after injury. n = 15 mice/group (Before injury) and 10 (Sham/Veh), 10 (Sham/PLX), 15 (SCI/Veh), and 15 (SCI/PLX) mice (After SCI). (D) Representative dot plots show the relative immune cell composition in the impact site of the spinal cord at two days post-injury. (E) The number of living CD45intCD11b+Ly6C- microglia, CD45hiCD11b+ infiltrating myeloid cells, CD45hiCD11b+Ly6C+Ly6G- monocytes, and CD45hiCD11b+Ly6C+Ly6G+ neutrophils are quantified. n = 6 (Sham/Veh), 6 (Sham/PLX), 7 (SCI/Veh), and 7 (SCI/PLX). (F) Representative histograms show the relative production of ROS in microglia, bulk infiltrating myeloid cells, monocytes, and neutrophils as measured by DHR123. (G) The mean fluorescence intensity (MFI) of dihydrorhodamine 123 (DHR123) was quantified for all cells. For all flow cytometry experiments. n = 6 (Sham/Veh), 6 (Sham/PLX), 7 (SCI/Veh), and 7 (SCI/PLX). (H) Quantification of the myeloid marker CD11b and the pro-inflammatory cytokine TNFα by qPCR analysis at 2 days post-injury. Total RNA was extracted from sham or injured spinal cord. n = 4 (Sham/Veh), 5 (Sham/PLX), 5 (SCI/Veh), and 6 (SCI/PLX). **p < 0.01, ***p < 0.001, ****p < 0.0001, ++++p < 0.0001, vs. Sham/Veh group. #p < 0.05, ##p < 0.01, ##p < 0.001, ####p < 0.0001, vs. SCI/Veh group. 2-way ANOVA following Tukey's multiple comparisons test.