Abstract
Today, Coronavirus Disease 2019 (COVID-19) is a global public health emergency and vaccination measures to counter its diffusion are deemed necessary. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the etiological agent of the disease, unleashes a T-helper 2 immune response in those patients requiring intensive care. Here, we illustrate the immunological mechanism to train the immune system towards a more effective and less symptomatic T-helper 1 immune response, to be exploited against SARS-CoV-2.
Keywords: Coronavirus Disease 2019 (COVID-19), Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Corynebacterium parvum (C. parvum), Propionibacterium acnes (P. acnes), Cutibacterium acnes (C. acnes), Bacillus Calmette-Guérin (BCG), Hyaluronic acid, cluster of differentiation 44 (CD44), T helper 1 (Th1), T helper 2 (Th2), Vaccine
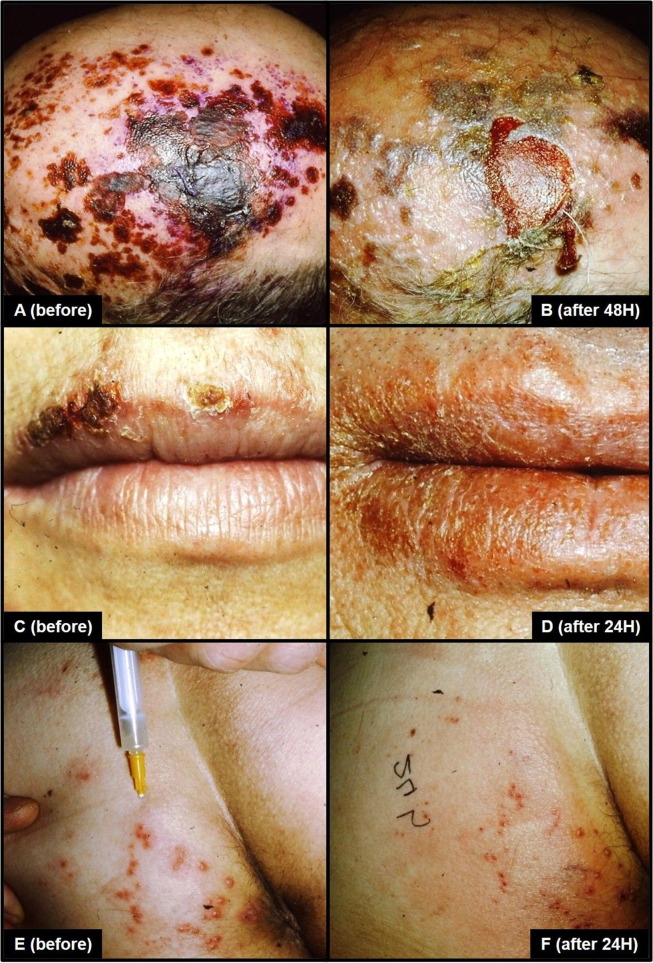
In the 60–80 years of last century, an experimental wave of immunological studies attempted to fight cancer by exploiting live or killed bacteria, among which the most investigated were the Bacillus Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, and Corynebacterium parvum (C. parvum), later renamed Propionibacterium acnes and then Cutibacterium acnes [1], [2]. Administered percutaneously or into the neoplastic mass, they proved able to induce the tumor lysis at some extent, and to delay or arrest the cancer growth through the innate immunity potentiation [3], [4]. BCG gained approval since 1977 and is currently the standard of care for patients with non-muscle-invasive bladder cancer by means of mucosal instillation, besides to be registered as an anti-tuberculosis intradermal vaccination [5], [6]. As of March 2020, BCG vaccine is furthermore in phase III or IV trials to prevent Coronavirus Disease 2019 (COVID-19) among healthcare workers in the Netherlands, Australia, USA, Germany, France, Denmark, Colombia, Mexico, Egypt, and South Africa [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. Additional trials in the Netherlands, Germany, Greece, and India are evaluating whether BCG vaccine provides protection against COVID-19 in the elderly and in middle age [17], [18], [19], [20]; besides, randomized trials on volunteers over 18 years to test BCG vaccine in this context has been launched in Brazil and Canada [21], [22]. C. parvum is an aerotolerant anaerobic rod-shaped Gram-positive bacterium largely commensal and part of the skin flora present on most healthy adults, but also associated to sarcoidosis and juvenile acne, hence its taxonomic renaming [23]. After an initial registration like immunoadjuvant and immunomodulator, C. parvum was added to the chemotherapy protocol for colon cancer by repeated injections in the form of formalin-killed freeze-dried vaccine preparation (Coparvax®, Wellcome Research Laboratories, Beckenham, UK); however, it was discarded because no partial remission, overall survival or significant benefits were achieved in the treated cohorts of patients [24]. At that time, one of us (Prof. Palmieri) had the chance to perform a clinical pilot trial with C. parvum administration into subcutaneous and lymph nodal metastases from lungs, thyroid and breast malignancies, noting local shrinking and colliquative effect in 48–72 h, accompanied by mild symptoms and occasional febrile peaks. In a few cases, very rapid regressions of concomitant herpes infections involving the head, the thorax and the genitals were incidentally observed. By searching on the English biomedical literature, several studies from the past fully support the C. parvum antiviral power on man and animal against, for example, influenza virus, hepatitis B, rabies, encephalomyocarditis virus, herpes zoster and human papilloma virus [25], [26], [27], [28], [29], [30]. In addition, a scientific evidence of C. parvum vaccine protection against a coronavirus (mouse hepatitis virus type 3), dating back to 1981 murine model by Schindler and colleagues, is also reported [31]. Always working on a murine model, Teixeira and collaborators proved in 2018 that C. parvum enhances the immunogenicity of the HIVBr18 vaccine, a vaccine against 18 epitopes of the human immunodeficiency virus, subtype B [32]. In the same year, Hsu and coworkers discovered 16 short RNA sequences from C. parvum similar to Ebola virus microRNAs, capable to protect the human host by influencing the thrombospondin 4 expression, a multifunctional protein which plays also an initiating role towards cell-mediated immunity in the skin [33]. Palmieri dropped out his clinical trial on bacterial immune stimulation against cancer in 1980, but he followed up with anecdotical compassionate treatments of severe herpes, and others heavy viral infections, such as influenza, mumps, varicella and measles, by subcutaneous injections of C. parvum cultured and killed in our microbiological lab, after signing an informed consent out of a total of 40 patients, 30 males and 10 females, aged between 5 and 97 years (mean age 49 years). During our evolving experience, we modified the subdermal injection formula (9 × 109 phenol-killed bacteria in 2 ml saline), adding high molecular weight hyaluronic acid as slow delivery system, having found aspecific virucide properties of this non-sulphated glycosaminoglycan, notoriously able to bind the cluster of differentiation 44 (CD44) receptors present on the surface of activated leukocytes [34], [35]. The protocol varied from 1 single to 3–5 injections each other day or every 3 days accordingly with the clinical stage and response to the treatment. The C. parvum administration has been always safe and quickly effective showing clinical improvement after 24, 48, 72, 96 h, depending on the patient’s performance status and the latency time between the infection outbreak and the beginning of the treatment (Fig. 1 ). As well known, naїve T-helper (Th0) cells can respond to novel infectious agents never encountered before, like the specific case of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the newly identified betacoronavirus responsible for COVID-19, able to bind the angiotensin converting enzyme 2 receptors [36]. On the basis of the encountered pathogen, Th0 then polarize the immune response into T-helper 1 (Th1), the default response in immunocompetent subjects to intracellular or phagocytosable pathogens (e.g. viruses, bacteria, protozoa, fungi), mediated by macrophages and T-cytotoxic (Tc) cells (cell-mediated immunity), or into T-helper 2 (Th2), classically directed against extracellular non-phagocytosable pathogens (e.g. helminths), whose main effectors are eosinophils, basophils, mastocytes and B cells (humoral immunity) [37]. In spite of this, severe SARS-CoV-2 infections are associated with marked Tc lymphopenia [38]. During our researches on COVID-19, we have disclosed that the immune system is forced to mount in critically ill patients a Th2 response, the only one still mountable in the attempt to counteract the viral load, rather than a Th1 response, which would keep the infection under control by means of macrophages and Tc cells [39], [40]. Moreover, for the first time in worldwide literature, we have provide evidence that a life-threatening escalation from Th2 immune response to type 3 hypersensitivity (immune complex disease) in COVID-19 vasculitis takes place, and that the inflamed smooth muscle cells of blood vessels concur to the «cytokine storm» via interleukin (IL)-6 [41]. Therefore, we have proposed that an effective vaccination strategy should be able to prevent or limit the systemic imbalance of Th2 cytokines [42], inducing a protective Th1 response to be exploited against SARS-CoV-2 (Fig. 2 ). Among the Th2 cytokines, there are IL-4, IL-5, IL-6, IL-9, IL-10, IL-13 and IL-25, while IL-2, IL-12, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) are the master Th1 cytokines [37]. Many researches have ascertained that C. parvum subcutaneous injection is able to induce a strong Th1 response favoring the production of IL-2, IL-12, IFN-γ and TNF-α, in practice as BCG works, and that the characteristic allergic Th2 response can be counterbalanced by C. parvum vaccination [43], [44], [45], [46], [47], [3], [48]; besides, it has been found a natural killers (NK) and dendritic cells activator [49], [50], [51]. In 1985 Cioffi and colleagues reported that C. parvum protects splenectomized Sprague Dawley rats from respiratory challenge with Streptococcus pneumoniae, without alter the number or activity of lavageable alveolar macrophages, and they hypothesized that C. parvum protection is more likely due to anincreasedclearance of blood-borne bacteria by the expanded and enhanced reticuloendothelial system [52]. If we transfer this murine model to man, a Th1 cytokines release syndrome from activated pulmonary macrophages after C. parvum lysate subcutaneous injection, such as to aggravate a possible superimposed COVID-19, appears somewhat unlikely. Therefore, our long-standing experience lays the foundation to revalue C. parvum lysate as a further surrogate vaccine against COVID-19, in the attempt to prevent or mitigate the cumbersome pandemic morbidity and mortality. The accurate preparation of the lysate is a crucial time to avoid opportunistic bone implant-associated infections or acute septic polyarthritis, whose a single case has been described in 1983 after C. parvum instillation for malignant pleural effusion; an episode of prolonged fever from immune system hyperactivation has also been reported [53], [54], [55]. Previous efforts to develop subunit vaccines against the most lethal human coronaviruses, for instance the Severe Acute Respiratory Syndrome Coronavirus 1 (SARS-CoV-1) and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), have failed because not enough protective; a recent study on SARS-CoV-2 has confirmed that neutralizing antibodies decline during the weeks or 2–3 months following infection at least in asymptomatic or paucisymptomatic patients [56], a biological behavior comparable to that of other known human coronaviruses [57], and which complicates the road to develop a specific long-term protective COVID-19 vaccine. By boosting Th1 response and innate immunity rather than the humoral one, the disappearance of neutralizing antibodies and the adaptive mutational potential of SARS-CoV-2, limiting factors for the development of an effective subunit vaccine, could be so circumvent. In this regard, a further recent study on 10.022 SARS-CoV-2 genomes has identified 5.775 distinct genome variants, including 2.969 missense mutations, 1.965 synonymous mutations, 484 mutations in the non-coding regions, 142 non-coding deletions, 100 in-frame deletions, 66 non-coding insertions, 36 stop-gained variants, 11 frameshift deletions and 2 in-frame insertions, a series of genetic events which determine SARS-CoV-2 virulence, infectivity and transmissibility [58]. In conclusion, we have here illustrated our rationale for a strategic off-target vaccination against SARS-CoV-2, promptly available and safe for the patients.
Fig. 1.
Illustrative clinical photographs taken by Prof. Palmieri before and after C. parvum vaccination: herpes zoster of the scalp pre-injection (A) and 48 h post-vaccine (B); herpes labialis before the injection (C) and after 24 h from the treatment (D); genital herpes at the time of subdermal injection (E) and 24 h post-vaccination (F).
Fig. 2.
Illustrative scheme of our vaccination rationale: following SARS-CoV-2 entry (point 1), the immune system is forced to mount a Th2 response in those patients requiring intensive care, at the expense of a more effective and less symptomatic Th1 response, compromised by the viral load (point 2). Through C. parvum or BCG vaccine (point 3), it is theoretically possible to train and calibrate the immune system towards a Th1 response (point 4), able to prevent COVID-19 or to keep the disease under control in a paucisymptomatic or asymptomatic way thanks to activated reticuloendothelial system, NK, Tc and dendritic cells.
Funding
None.
Authors contribution
LR conceived, designed and supervised the study, prepared figures and related legends, revised critically the first draft and wrote the final version of the manuscript; MV and VC analyzed and interpreted the data and performed the literature search; BP conceived and designed the study and wrote the first draft of the manuscript. All the authors approved the final version of the manuscript and attested they meet the ICMJE criteria for authorship.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Oettgen H.F. Immunotherapy of cancer. N Engl J Med. 1977;297:484–491. doi: 10.1056/NEJM197709012970907. [DOI] [PubMed] [Google Scholar]
- 2.Lipton A., Harvey H.A., Lawrence B., Gottlieb R., Kukrika M., Dixon R. Corynebacterium parvum versus BCG adjuvant immunotherapy in human malignant melanoma. Cancer. 1983;51:57–60. doi: 10.1002/1097-0142(19830101)51:1<57::aid-cncr2820510114>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Tchaptchet S., Kirberg J., Freudenberg N., Schamel W.W., Galanos C., Freudenberg M.A. Innate, antigen-independent role for T cells in the activation of the immune system by Propionibacterium acnes. Eur J Immunol. 2010;40:2506–2516. doi: 10.1002/eji.200939860. [DOI] [PubMed] [Google Scholar]
- 4.Angelidou A., Conti M.G., Diray-Arce J., Benn C.S., Shann F., Netea M.G. Licensed Bacille Calmette-Guérin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine. 2020;38:2229–2240. doi: 10.1016/j.vaccine.2019.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization BCG vaccines: WHO position paper – February 2018. Wkly Epidemiol Rec. 2018;93:73–96. [Google Scholar]
- 6.Brandau S., Suttmann H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed Pharmacother. 2007;61:299–305. doi: 10.1016/j.biopha.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 7.U.S. National Library of Medicine. Reducing health care workers absenteeism in Covid-19 pandemic through BCG vaccine (BCG-CORONA). ClinicalTrials.gov identifier: NCT04328441; 2020 [last accessed April 29, 2020].
- 8.U.S. National Library of Medicine. BCG vaccination to protect healthcare workers against COVID-19 (BRACE). ClinicalTrials.gov identifier: NCT04327206; 2020 [last accessed July 8, 2020].
- 9.U.S. National Library of Medicine. BCG vaccine for health care workers as defense against COVID-19 (BADAS). ClinicalTrials.gov identifier: NCT04348370; 2020 [last accessed May 27, 2020].
- 10.U.S. National Library of Medicine. Study to assess VPM1002 in reducing healthcare professionals' absenteeism in COVID-19 pandemic. ClinicalTrials.gov identifier: NCT04387409; 2020 [last accessed June 16, 2020].
- 11.U.S. National Library of Medicine. Efficacy of BCG vaccination in the prevention of COVID19 via the strengthening of innate immunity in health care workers (COVID-BCG). ClinicalTrials.gov identifier: NCT04384549; 2020 [last accessed May 12, 2020].
- 12.U.S. National Library of Medicine. Using BCG vaccine to protect health care workers in the COVID-19 pandemic. ClinicalTrials.gov identifier: NCT04373291; 2020 [last accessed May 5, 2020].
- 13.U.S. National Library of Medicine. Performance evaluation of BCG vaccination in healthcare personnel to reduce the severity of SARS-COV-2 infection. ClinicalTrials.gov identifier: NCT04362124; 2020 [last accessed April 28, 2020].
- 14.U.S. National Library of Medicine. Prevention, efficacy, and safety of BCG vaccine in COVID-19 among healthcare workers. ClinicalTrials.gov identifier: NCT04461379; 2020 [last accessed July 14, 2020].
- 15.U.S. National Library of Medicine. Application of BCG vaccine for immune-prophylaxis among Egyptian healthcare workers during the pandemic of COVID-19. ClinicalTrials.gov identifier: NCT04350931; 2020 [last accessed April 20, 2020].
- 16.U.S. National Library of Medicine. BCG vaccination for healthcare workers in COVID-19 pandemic. ClinicalTrials.gov identifier: NCT04379336; 2020 [last accessed May 7, 2020].
- 17.U.S. National Library of Medicine. Reducing COVID-19 related hospital admission in elderly by BCG vaccination. ClinicalTrials.gov identifier: NCT04417335; 2020 [last accessed June 4, 2020].
- 18.U.S. National Library of Medicine. Study to assess VPM1002 in reducing hospital admissions and/or severe respiratory infectious diseases in elderly in COVID-19 pandemic. ClinicalTrials.gov identifier: NCT04435379; 2020 [last accessed August 6, 2020].
- 19.U.S. National Library of Medicine. Bacillus Calmette-Guérin vaccination to prevent COVID-19 (ACTIVATEII). ClinicalTrials.gov identifier: NCT04414267; 2020 [last accessed July 14, 2020].
- 20.U.S. National Library of Medicine. BCG vaccine in reducing morbidity and mortality in elderly individuals in COVID-19 hotspots. ClinicalTrials.gov identifier: NCT04475302; 2020 [last accessed July 23, 2020].
- 21.U.S. National Library of Medicine. COVID-19: BCG as therapeutic vaccine, transmission limitation, and immunoglobulin enhancement (BATTLE). ClinicalTrials.gov identifier: NCT04369794; 2020 [last accessed August 7, 2020].
- 22.U.S. National Library of Medicine. Efficacy and safety of VPM1002 in reducing SARS-CoV-2 (COVID-19) infection rate and severity (COBRA). ClinicalTrials.gov identifier: NCT04439045; 2020 [last accessed June 19, 2020].
- 23.Palmieri B., Vadalà M., Roncati L., Garelli A., Scandone F., Bondi M. The long-standing history of Corynebacterium parvum, immunity, and viruses. J Med Virol. 2020 doi: 10.1002/jmv.26100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whisnant J.K. C. parvum clinical protocols: prototypes and summary results in U.S. trials with Wellcome Coparvax. Dev Biol Stand. 1977;38:559–566. [PubMed] [Google Scholar]
- 25.Mak N.K., Schiltknecht E., Ada G.L. Protection of mice against influenza virus infection: enhancement of nonspecific cellular responses by Corynebacterium parvum. Cell Immunol. 1983;78:314–325. doi: 10.1016/0008-8749(83)90286-1. [DOI] [PubMed] [Google Scholar]
- 26.Papaevangelou G., Sparros L., Vissoulis C., Kyriakidou A., Giokas G., Hadzimanolis J. The effect of intradermal administration of Corynebacterium parvum on the immune response to hepatitis Bs antigen. J Med Virol. 1977;1:15–19. doi: 10.1002/jmv.1890010104. [DOI] [PubMed] [Google Scholar]
- 27.Megid J., Kaneno R., Nozaki C.N., Brito C.J., Almeida M.F. Increased interleukin-10 associated with low IL-6 concentration correlated with greater survival rates in mice infected by rabies virus vaccinated against it and immunomodulated with P. acnes. Comp Immunol Microbiol Infect Dis. 2004;27:393–411. doi: 10.1016/j.cimid.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Géniteau-Legendre M., Forestier F., Quéro A.M., German A. Role of interferon, antibodies and macrophages in the protective effect of Corynebacterium parvum on encephalomyocarditis virus-induced disease in mice. Antiviral Res. 1987;7:161–167. doi: 10.1016/0166-3542(87)90004-0. [DOI] [PubMed] [Google Scholar]
- 29.Topciu V., Mihăilescu R. Immunomodulating and antiviral therapy in herpes zoster. Rom J Virol. 1996;47:75–80. [PubMed] [Google Scholar]
- 30.Nasser N. Treatment of common warts with the immune stimulant Propionium bacterium parvum. An Bras Dermatol. 2012;87:585–589. doi: 10.1590/s0365-05962012000400011. [DOI] [PubMed] [Google Scholar]
- 31.Schindler L., Streissle G., Kirchner H. Protection of mice against mouse hepatitis virus by Corynebacterium parvum. Infect Immun. 1981;32:1128–1131. doi: 10.1128/iai.32.3.1128-1131.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teixeira D., Ishimura M.E., Apostólico J.S., Viel J.M., Passarelli V.C., Cunha-Neto E. Propionibacterium acnes enhances the immunogenicity of HIVBr 18 human immunodeficiency virus-1 vaccine. Front Immunol. 2018;9:177. doi: 10.3389/fimmu.2018.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu P.C., Chiou B.H., Huang C.M. On revealing the gene targets of Ebola virus microRNAs involved in the human skin microbiome. PeerJ. 2018;6 doi: 10.7717/peerj.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cermelli C., Cuoghi A., Scuri M., Bettua C., Neglia R.G., Ardizzoni A. In vitro evaluation of antiviral and virucidal activity of a high molecular weight hyaluronic acid. Virol J. 2011;8:141. doi: 10.1186/1743-422X-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aruffo A., Stamenkovic I., Melnick M., Underhill C.B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 36.Roncati L., Gallo G., Manenti A., Palmieri B. Renin-angiotensin system: the unexpected flaw inside the human immune system revealed by SARS-CoV-2. Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spellberg B., Edwards J.E., Jr. Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Deng Y. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis. 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roncati L., Nasillo V., Lusenti B., Riva G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann Hematol. 2020;99:1419–1420. doi: 10.1007/s00277-020-04066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roncati L., Lusenti B. The «moonlighting protein» able to explain the Th1 immune lockdown in severe COVID-19. Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roncati L., Ligabue G., Fabbiani L., Malagoli C., Gallo G., Lusenti B. Type 3 hypersensitivity in COVID-19 vasculitis. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roncati L., Palmieri B. What about the original antigenic sin of the humans versus SARS-CoV-2? Med Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kistowska M., Meier B., Proust T., Feldmeyer L., Cozzio A., Kuendig T. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110–118. doi: 10.1038/jid.2014.290. [DOI] [PubMed] [Google Scholar]
- 44.Furusawa H., Suzuki Y., Miyazaki Y., Inase N., Eishi Y. Th1 and Th17 immune responses to viable Propionibacterium acnes in patients with sarcoidosis. Respir Investig. 2012;50:104–109. doi: 10.1016/j.resinv.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Tsuda K., Yamanaka K., Linan W., Miyahara Y., Akeda T., Nakanishi T. Intratumoral injection of Propionibacterium acnes suppresses malignant melanoma by enhancing Th1 immune responses. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0029020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girvan R.C., Knight D.A., O'Loughlin C.J., Hayman C.M., Hermans I.F., Webster G.A. MIS416, a non-toxic microparticle adjuvant derived from Propionibacterium acnes comprising immunostimulatory muramyl dipeptide and bacterial DNA promotes cross-priming and Th1 immunity. Vaccine. 2011;29:545–557. doi: 10.1016/j.vaccine.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 47.Kitagawa H., Yamanaka K., Kakeda M., Inada H., Imai Y., Gabazza E.C. Propionibacterium acnes vaccination induces regulatory T cells and Th1 immune responses and improves mouse atopic dermatitis. Exp Dermatol. 2011;20:157–158. doi: 10.1111/j.1600-0625.2010.01180.x. [DOI] [PubMed] [Google Scholar]
- 48.O'Neill L.A.J., Netea M.G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ananias R.Z., Rodrigues E.G., Braga E.G., Squaiella C.C., Mussalem J.S., Longhini A.L. Modulatory effect of killed Propionibacterium acnes and its purified soluble polysaccharide on peritoneal exudate cells from C57Bl/6 mice: major NKT cell recruitment and increased cytotoxicity. Scand J Immunol. 2007;65:538–548. doi: 10.1111/j.1365-3083.2007.01939.x. [DOI] [PubMed] [Google Scholar]
- 50.Balch C.M., Smalley R.V., Bartolucci A.A., Burns D., Presant C.A., Durant J.R. A randomized prospective clinical trial of adjuvant C. parvum immunotherapy in 260 patients with clinically localized melanoma (Stage I): prognostic factors analysis and preliminary results of immunotherapy. Cancer. 1982;49:1079–1084. doi: 10.1002/1097-0142(19820315)49:6<1079::aid-cncr2820490604>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 51.Hirt H.M., Schwenteck M., Becker H., Kirchner H. Interferon production and lymphocyte stimulation in human leucocyte cultures stimulated by Corynebacterium parvum. Clin Exp Immunol. 1978;32:471–476. [PMC free article] [PubMed] [Google Scholar]
- 52.Cioffi W.G., Hebert J.C., Gamelli R.L., Foster R.S., Jr. The quantity and function of pulmonary alveolar macrophages after splenectomy and Corynebacterium parvum. J Trauma. 1985;25:405–409. doi: 10.1097/00005373-198505000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Dubus M., Varin J., Papa S., Rammal H., Chevrier J., Maisonneuve E. Interaction of Cutibacterium acnes with human bone marrow derived mesenchymal stem cells: a step toward understanding bone implant-associated infection development. Acta Biomater. 2020;104:124–134. doi: 10.1016/j.actbio.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 54.Lever A.M., Forsythe J., Oxford P. Acute polyarthritis following the use of Corynebacterium parvum vaccine (Coparvax) for malignant pleural effusion. Postgrad Med J. 1983;59:799–800. doi: 10.1136/pgmj.59.698.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laroche C.M., Britton M. Prolonged fever after pleural instillation of Corynebacterium parvum (Coparvax) Thorax. 1987;42:823–824. doi: 10.1136/thx.42.10.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020 doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 57.Reed S.E. The behaviour of recent isolates of human respiratory coronavirus in vitro and in volunteers: evidence of heterogeneity among 229E-related strains. J Med Virol. 1984;13:179–192. doi: 10.1002/jmv.1890130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koyama T., Platt D., Parida L. Variant analysis of SARS-CoV-2 genomes. Bull World Health Organ. 2020;98:495–504. doi: 10.2471/BLT.20.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]