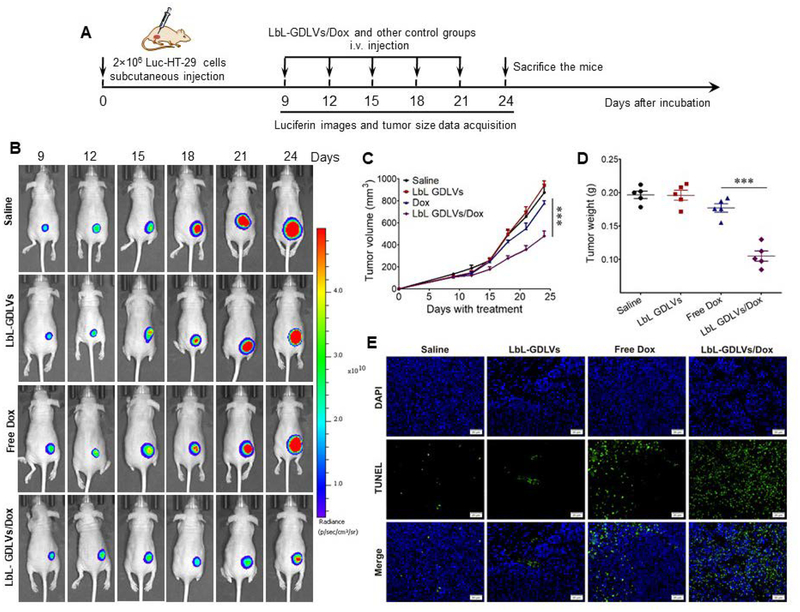
Figure 6. LbL-GDLVs/Dox have in vivo therapeutic efficacy in Luc-HT-29 tumor-bearing mice.
A. Illustration of the experimental design. Luc-HT-29 tumor-bearing mice were established, randomly divided into four groups, and treated with the LbL-GDLVs/Dox or controls consisting of saline, LbL-GDLVs, and free Dox (n=5). B. Representative bioluminescence images of mice following the administration of luciferin substrate. On days (D)9, D12, D15, D18, D21 and D24 post-injection of Luc-HT-29, tumor cells were visualized using an in vivo imaging system (n=5). C. Tumor growth profiles in mice treated with LbL-GDLV/Dox or control formulations. Data are presented as the mean value ± SD (n=5); ***p<0.001. D. Tumor weights in each group were measured at the end of the experiment (n=5); ***p<0.001. E. Terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) assay was used to evaluate the levels of apoptotic cells (green) in Luc-HT-29 tumors (n=5). Scale bar: 20 μm.