Fig. 6 |. Plasticity of the SCP structure and implications for tegument protein recruitment.
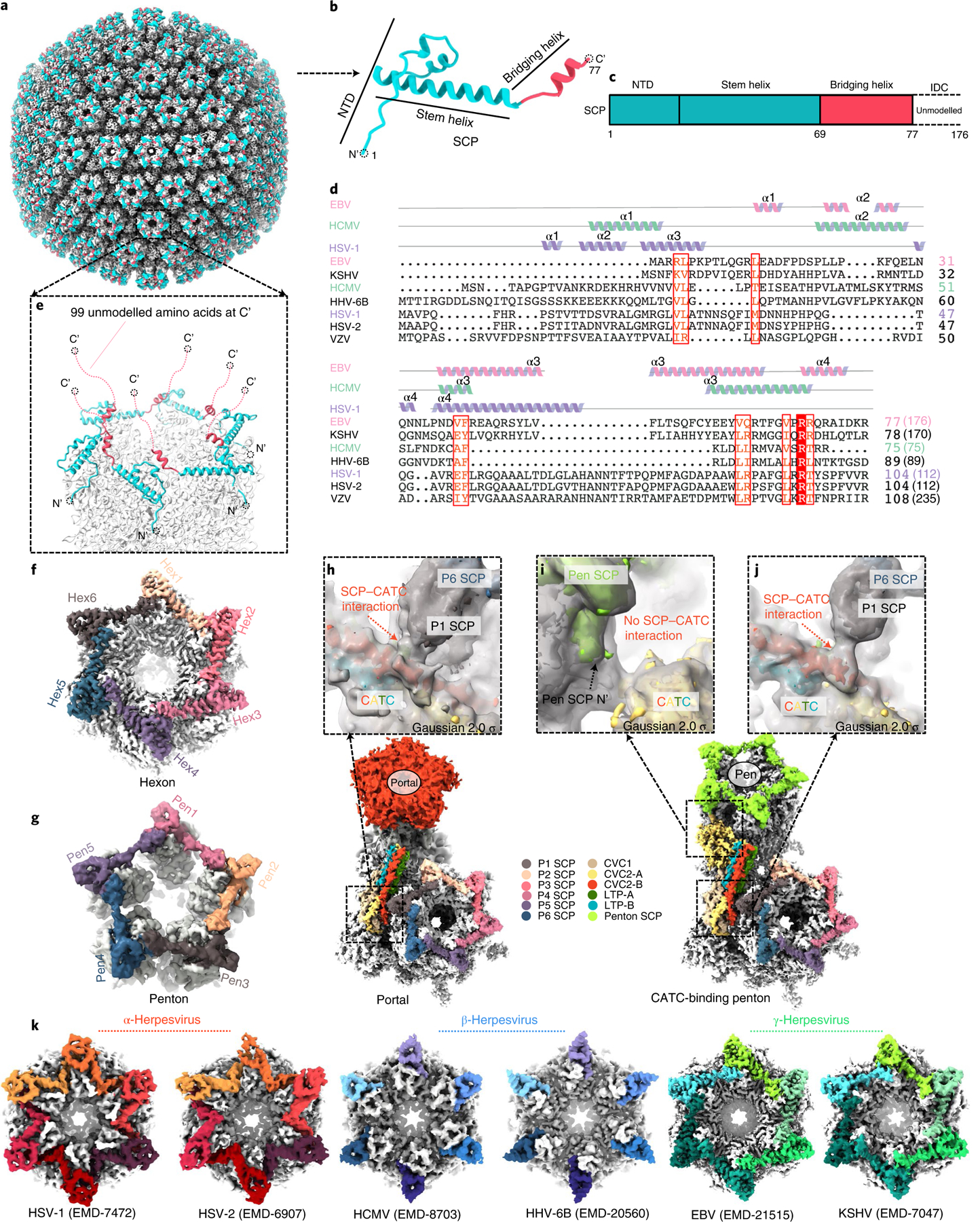
a–g, Structure of the eBV SCP in the penton and hexon. The SCP has a helix-rich N-terminal half (b) that sits on top of both the penton and the hexon of the capsid (a), bridging adjacent MCP subunits (e–g) and the flexible C-terminal half emanating into the tegument layer (e). c, Schematic diagram of the domain organization of SCP. Structure and sequence alignments (d) indicate that eBV SCP differs from known SCP structures. Lengths of SCP sequences are indicated in parentheses. ICD, intrinsically disordered C-terminal domain; VZV, varicella-zoster virus; HHV, human herpesvirus. f–g, Representative eBV hexon (f) and penton (g). h–j, Interactions between CATC and hexon SCP near the portal vertex (h) and the penton vertex (i and j). k, Comparison of interactions between SCP (colour) and MCP (grey) in the hexons of three subfamilies of herpesviruses.
