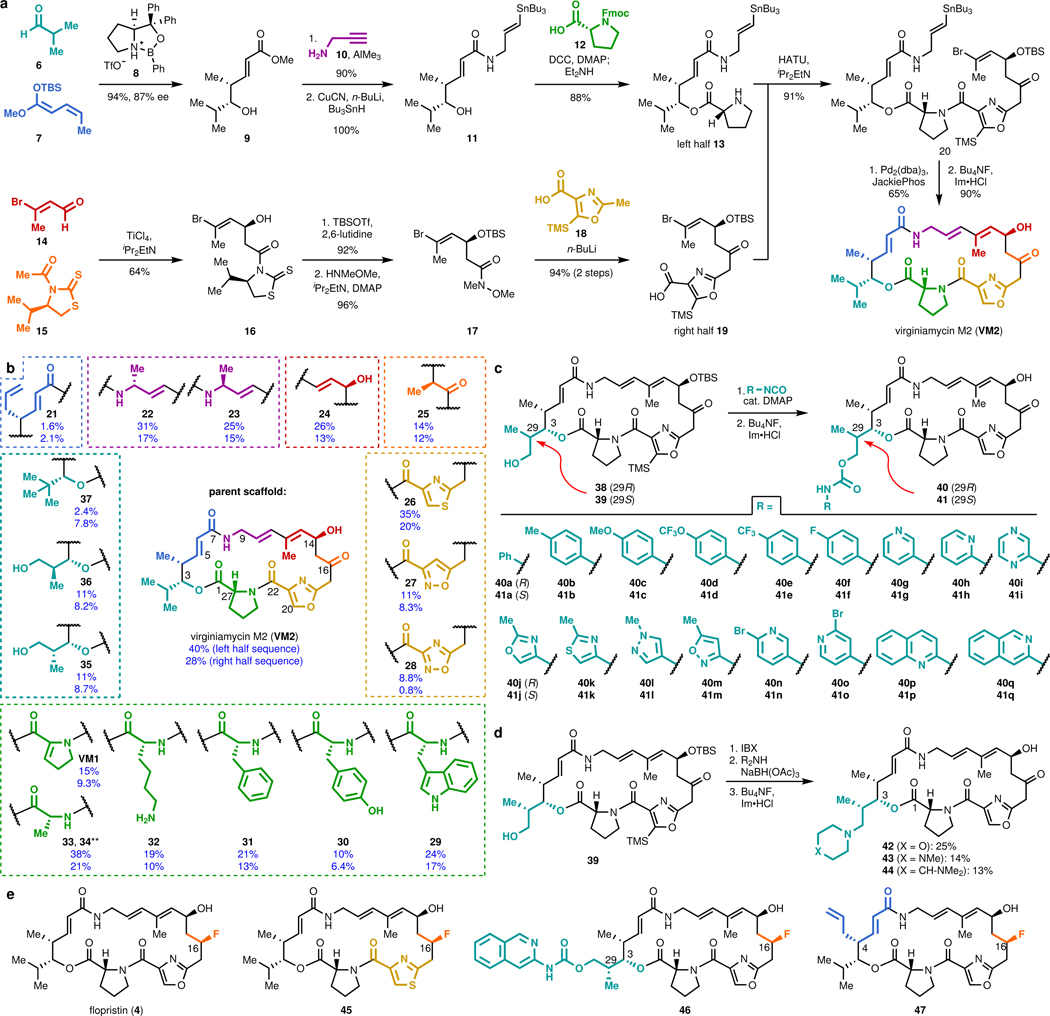
Figure 1 |. Modular synthesis enables access to >60 fully synthetic group A streptogramins.
a, Convergent route to VM2 from seven building blocks. b, Eighteen group A streptogramins accessed by building block variation. The fragments displayed in the dashed boxes represent the structural variability compared to the parent scaffold (VM2). Overall yields for the synthesis of each analog for the left half sequence (top number) and for the right half sequence (bottom number) are displayed. **Instead of a ketone, madumycin II (34) contains the following substitution at C16: ɑ-H, β-OH. c, Access to 34 analogs (17 in each diastereomeric series) with C3 side chain variability by means of carbamate formation followed by desilylation. d, Synthesis of tertiary-amine-containing analogs by oxidation and reductive amination. e, C16-fluorinated analogs.