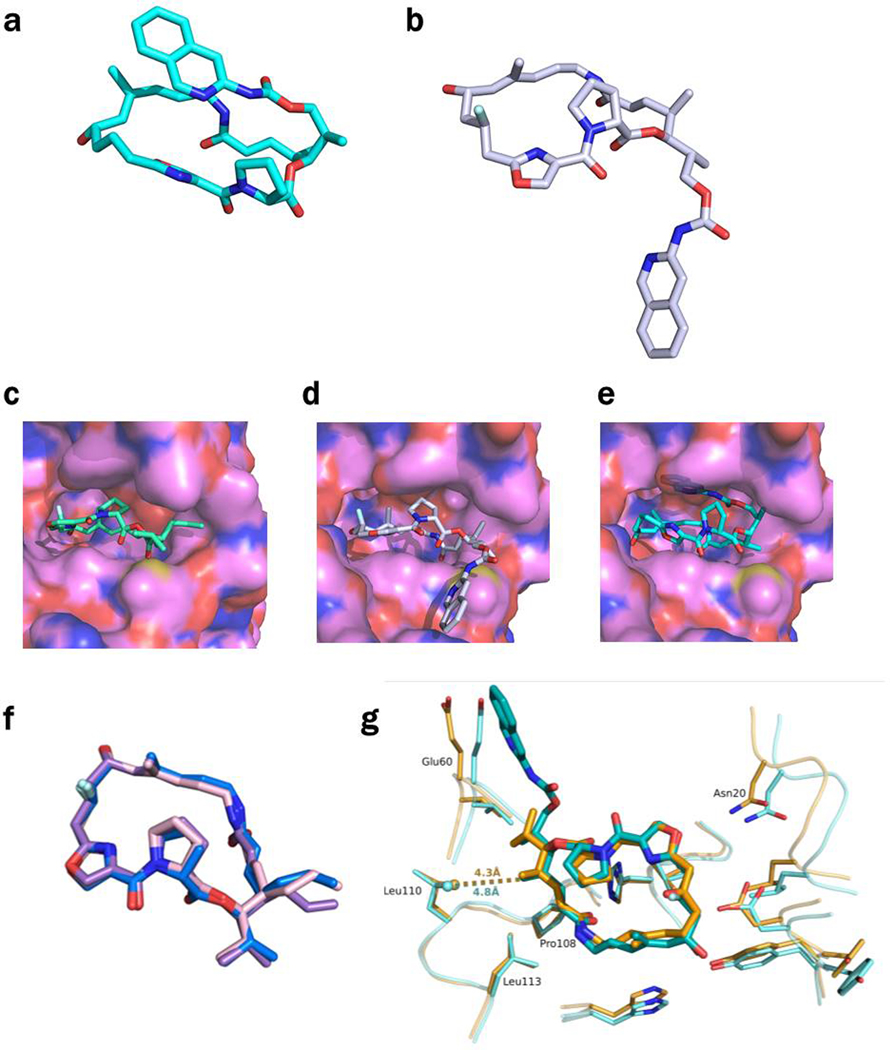
Extended Data Figure 7 -. Conformations of 46 and 47 in the ribosome and in VatA.
a, The conformation of 46 minimized by QM methods in low dielectric, shows how the isoquinoline side chain packs over the macrocycle. b, In contrast, the ribosome-bound conformations of 46 determined by CryoEM show that the side chain extends away from the macrocycle due to interactions formed in the binding site. c, Model of 47 in the conformation bound to the ribosome modeled into the active site of VatA (shown in surface). d, Model of 46 in the conformation bound to the ribosome modeled into the active site of VatA. e, Low energy model of 46 modeled into the active site of VatA. f, Overlay of VatA-bound (marine), ribosome-bound (violet), and ribosome with VS1-bound (light pink) conformations of 47. g, X-ray crystal structures of VM1 bound to VatA (PDB ID: 4HUS, 2.4 Å) and 46 bound to VatA at 2.8-Å resolution.