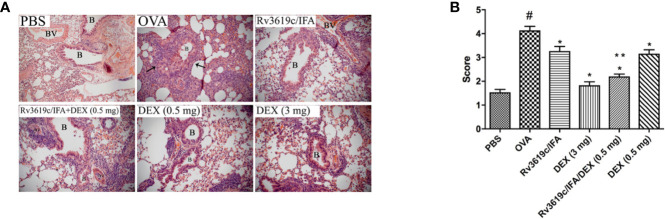
Figure 3.
A representative low-magnification light photomicrographs (A) and score (B) display H&E staining of whole lung samples from PBS-challenged/vehicle, ovalbumin (OVA)-challenged/vehicle, OVA-challenged/Rv3619c/IFA, OVA-challenged/dexamethasone (DEX) (3 mg), OVA-challenged/Rv3619c/IFA/dexamethasone (DEX) (0.5 mg), and OVA-challenged/dexamethasone (DEX) (0.5 mg). OVA-challenged/vehicle treated mice had marked peribronchial and perivascular inflammatory cell infiltrations compared with PBS-challenged/vehicle treated mice. Immunization with Rv3619c/IFA alone resulted in a significant reduction in the peribronchial and perivascular inflammatory cell infiltration compared to OVA-challenged/vehicle treated mice and was comparable with high dose dexamethasone (3 mg). Immunization with Rv3619c/IFA in combination with dexamethasone (0.5 mg) resulted in a greater inhibitory effect on the inflammatory response compared to immunization with Rv3619c/IFA alone. B = bronchioles, BV = blood vessels, (➞) = marked peribronchial and perivascular inflammatory cell infiltrations. Data are expressed as mean ± SEM (n = 10). #P < 0.05 versus time-matched PBS-challenged mice. *P < 0.05 versus time-matched OVA-challenged mice. **P < 0.05 versus Rv3619c/IFA and dexamethasone (0.5 mg).