Figure 7.
A Secondary Tigecycline Binding Site at the 50S Central Protuberance
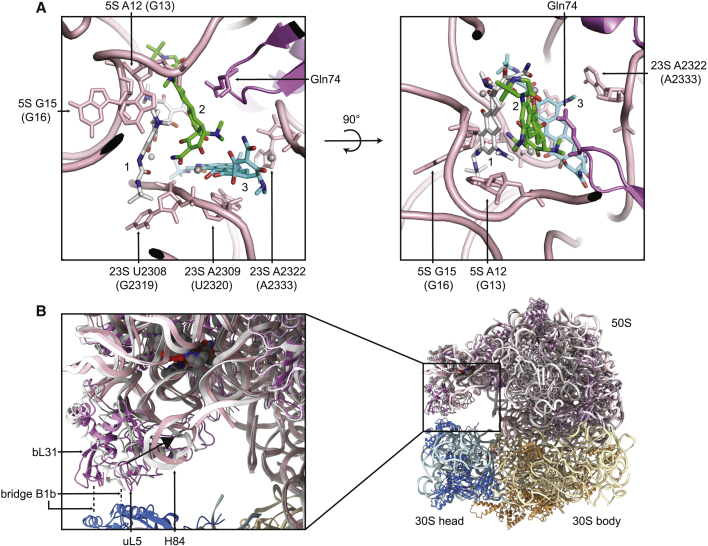
(A) Two views of the secondary binding site in the A. baumannii ribosome-tigecycline structure. The three tigecycline molecules are labeled 1 (white), 2 (green), and 3 (cyan). The 23S rRNA nucleotides U2308, U2309, and A2322; the 5S rRNA nucleotides A12 and G15; and the bL27 residue Gln74, which interact with the drug molecules, are labeled. Magnesium ions are shown as gray spheres.
(B) Changes in the conformation of the central protuberance and intersubunit bridge B1b upon tigecycline binding at this secondary site. The atomic model of the tigecycline-bound ribosome (50S pink, 30S body brown, 30S head blue) is overlaid with the atomic model of the amikacin-bound 50S, which has no tigecycline bound (white). H84 and uL5 undergo a shift upon tigecycline binding, and bL31 becomes partially resolved in the density.
E. coli numbering is shown in parentheses.
See also Figure S7.