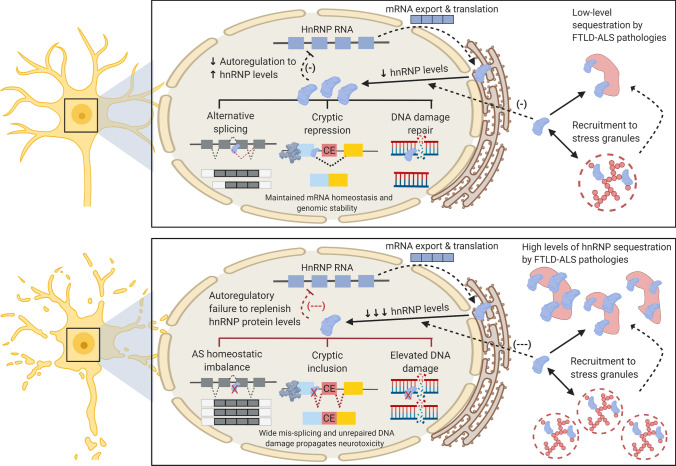
Fig. 5.
Proposed model of hnRNP dysfunction in FTLD-ALS. The upper panel illustrates hnRNPs continuing to perform their homeostatic functions under relatively low levels of stress e.g., at early stages of FTLD-ALS pathogenesis. HnRNP protein levels are reduced as a result of low-level sequestration within cytoplasmic pathological inclusions (nuclear inclusions not shown) and/or recruitment to stress granules. Indeed persistence of stress granules may be the root cause of some of these aggregates. However, autoregulation ensures adequate amounts of hnRNPs are replenished so they may perform their myriad nuclear functions including alternative splicing regulation, cryptic exon repression and DNA damage repair. By contrast, the lower panel illustrates a scenario whereby hnRNP depletion by pathological sequestration breaches a homeostatic ‘tipping point’ that is beyond compensation by autoregulatory means. At this stage, ensuing mRNA metabolic dysfunction from alternative splicing dysregulation and elevated cryptic exon activation in addition to unrepaired DNA damage may rapidly lead to neurotoxicity and accelerated neurodegeneration