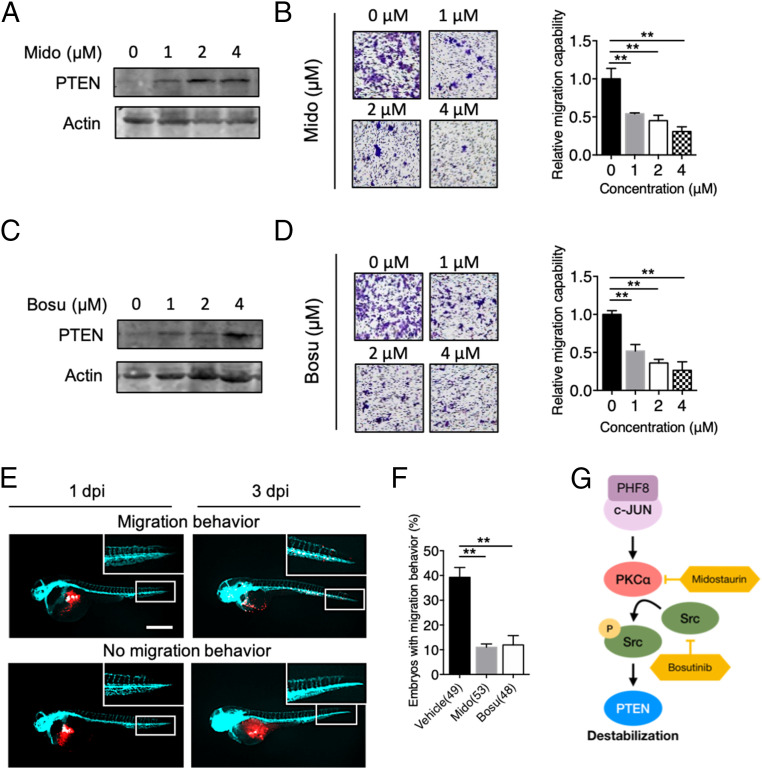
Fig. 6.
Pharmacological inhibition of PHF8-PKCα-Src-PTEN blocks GC progression in vitro and in vivo. (A) Analysis of PTEN protein level in MKN28 treated with 0, 1, 2, or 4 μM of midostaurin (Mido). (B) Detection of migration activity of MKN28 treated with Mido by using Transwell migration assay. (C) Analysis of PTEN protein level in MKN28 treated with 0, 1, 2, or 4 μM of bosutinib (Bosu). (D) Detection of migration activity of MKN28 treated with Bosu by using Transwell migration assay. In B and D, data are presented as the average of three replicates ± SD **P < 0.01 (two-tailed Student’s t test). (E and F) Zebrafish xenotransplantation assays using Mido or Bosu. MKN28 labeled with CM-Dil (red fluorescence dye in the membrane) were ectopically injected into the yolk-sac parts of 2-d-old Tg(fli1:EGFP) (fish with green fluorescence in blood vessels) zebrafish embryos, followed by immersion in solutions containing 1 μM midostaurin or bosutinib (a sublethal dose) at 1 dpi. Fluorescence microscopic analysis was conducted at 1 dpi and 3 dpi. Representative fluorescence images of a zebrafish embryo displaying cell dissemination (Upper) or no migration (Lower) at 3 dpi (E). Cyan, blood vessel. (Scale bar, 100 µm.) Quantification of embryos with migration behavior in vehicle or drug-treated groups (F). Total number of embryos used in vehicle, Mido, and Bosu is shown in the bracket. Data were obtained from three independent studies. **P < 0.01 (two-tailed Student’s t test). (G) A schematic diagram shows that the PHF8-c-Jun complex contributes to GC progression through activation of PKCα-Src-dependent signaling to suppress PTEN. Targeting the PHF8-c-Jun-PKCα-Src-PTEN axis represents a prognostic/therapeutic target in advanced GC.