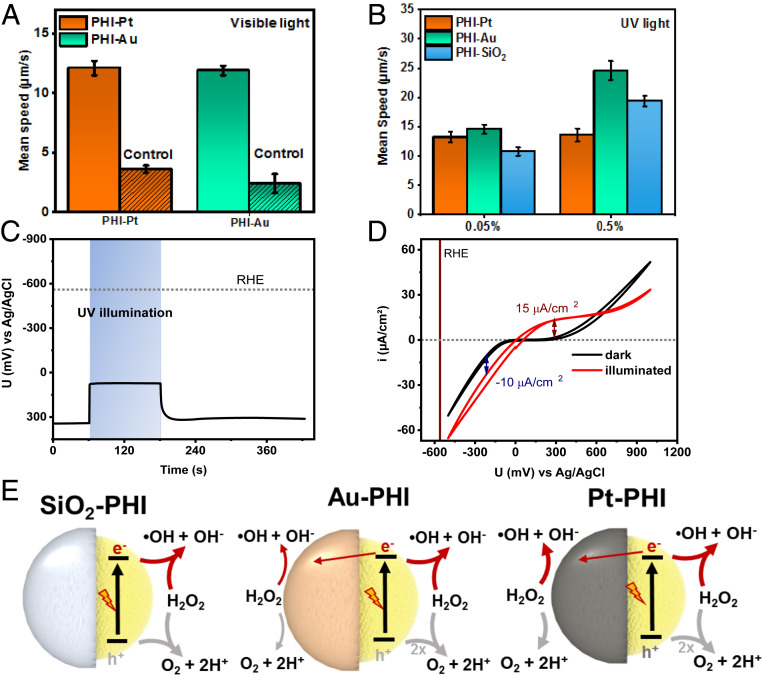
Fig. 3.
Characterization of the swimming behavior of PHI-based Janus microswimmers in H2O2. (A) Average (n = 25) mean speed of the visible light-driven PHI–Au and PHI–Pt microswimmers with 2% H2O2 as fuel and the control experiment in the dark. (B) Average (n = 25) mean speed of UV-light propelled PHI-based microswimmers with Pt, Au, and SiO2 caps at low H2O2 concentrations (0.05 and 0.5%) (Movies S4 and S5). (C) OCP measurement of PHI nanosheets deposited on FTO in DI water containing 1% H2O2 in the dark, under and after UV illumination. (D) CV in dark (black curve) and under UV illumination (red curve) with the same conditions as in C. (E) Schematics illustrating the (competing) major and minor redox reactions with H2O2 on both sides of the three PHI-based Janus microswimmers. Reduction reactions (driven by electrons) are indicated by red arrows, whereas the oxidation reactions are highlighted in gray. The electron transfer between the PHI and the metal cap (straight red arrow) indicates a possible self-electrophoretic contribution.