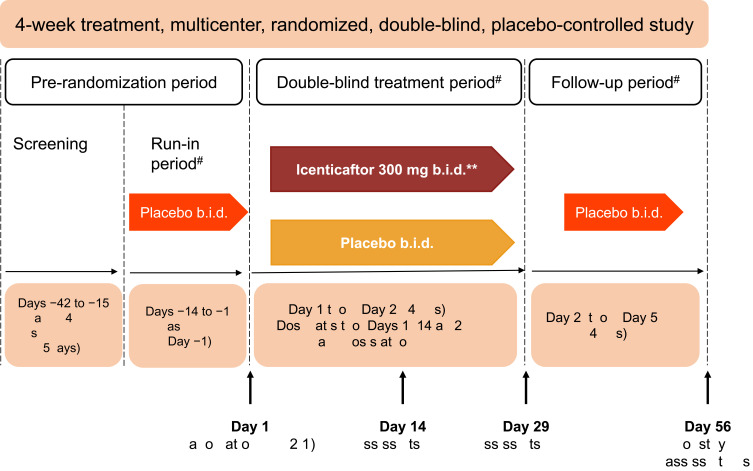
Figure 1.
Study design. *Primary efficacy assessments on Day 29–12 hours after the last dose of icenticaftor. **A total of 92 patients were randomized. The first 4 patients in the study received icenticaftor 450 mg b.i.d. or placebo, prior to the protocol amendments reducing the dose to icenticaftor 300 mg b.i.d. #Patients continued on background COPD therapy throughout the study. During the entire study duration, subjects were maintained on stable baseline COPD therapy.
Abbreviations: b.i.d., twice daily; EOS, end of study.