Abstract
Background
In polymorbid patients with bronchopulmonary infection, malnutrition is an independent risk factor for mortality. There is a lack of interventional data investigating whether providing nutritional support during the hospital stay in patients at risk for malnutrition presenting with lower respiratory tract infection lowers mortality.
Methods
For this secondary analysis of a randomized clinical trial (EFFORT), we analyzed data of a subgroup of patients with confirmed lower respiratory tract infection from an initial cohort of 2028 patients. Patients at nutritional risk (Nutritional Risk Screening [NRS] score ≥3 points) were randomized to receive protocol-guided individualized nutritional support to reach protein and energy goals (intervention group) or standard hospital food (control group). The primary endpoint of this analysis was all-cause 30-day mortality.
Results
We included 378 of 2028 EFFORT patients (mean age 74.4 years, 24% with COPD) into this analysis. Compared to usual care hospital nutrition, individualized nutritional support to reach caloric and protein goals showed a similar beneficial effect of on the risk of mortality in the subgroup of respiratory tract infection patients as compared to the main EFFORT trial (odds ratio 0.47 [95%CI 0.17 to 1.27, p = 0.136] vs 0.65 [95%CI 0.47 to 0.91, p = 0.011]) with no evidence of a subgroup effect (p for interaction 0.859). Effects were also similar among different subgroups based on etiology and type of respiratory tract infection and for other secondary endpoints.
Conclusion
This subgroup analysis from a large nutrition support trial suggests that patients at nutritional risk as assessed by NRS 2002 presenting with bronchopulmonary infection to the hospital likely have a mortality benefit from individualized inhospital nutritional support. The small sample size and limited statistical power calls for larger nutritional studies focusing on this highly vulnerable patient population.
Clinical trial registration
Registered under ClinicalTrials.gov Identifier no. NCT02517476.
Keywords: Malnutrition, Nutritional support, Outcome, Randomized trial, Respiratory infection, COVID19
1. Introduction
Respiratory tract infection poses an important risk to patients, particularly the polymorbid population is at highest risk for mortality and adverse outcome. The risk may further increase, if patients are malnourished upon hospital admission or if patients become malnourished during the hospital stay [1]. In the frail polymorbid patient population, malnutrition often develops slowly due to different chronic illnesses causing loss of appetite and cachexia due to disease-related anorexia and inflammation, loss of muscle mass and sarcopenia [[2], [3], [4], [5]]. Malnutrition can be found in up to 40% of hospitalized patients with community-acquired pneumonia and is associated with a 2.5-fold increase in mortality [6]. Similar data have also been reported for patients hospitalized for other types of respiratory infection including COPD exacerbation and COVID-19 infection with a high prevalence of malnutrition and worse outcomes in the malnourished population [7,8]. Specifically, observational research found strong associations of poor nutritional status with prolonged length of hospital stay and higher disease severity [9].
To reduce the burden of malnutrition-associated adverse outcomes, several international nutritional societies and expert committees have published recommendations for the prevention, diagnosis and treatment of malnutrition in medical patients in general, and for patients with lower respiratory tract infections due to COVID-19 in particular [8,[10], [11], [12], [13]]. However, recommendations regarding nutritional support in patients with lower respiratory tract infections are largely based on physiological rationales and observational studies, and extrapolated from interventional research looking at general medical inpatients [14,15]. However, interventional trials specifically focusing on patients with lower respiratory tract infections are currently largely lacking.
Herein, we performed a preplanned secondary analysis of the Effect of early nutritional support on Frailty, Functional Outcomes and Recovery of malnourished medical inpatients Trial (EFFORT) looking specifically at the population of patients with lower respiratory tract infections [16]. We tested the hypothesis that protocol-guided individualized nutritional support to reach protein and energy goals reduces the risk of mortality and other adverse clinical outcomes in the subgroup of hospitalized inpatients at nutritional risk with confirmed infection of the lung.
2. Materials and methods
2.1. Study design and patient population
This is a secondary analysis of the EFFORT trial, a pragmatic, investigator-initiated, open-label, non-blinded, multicenter, randomized, controlled trial. The trial protocol and the main results were previously published elsewhere [[16], [17], [18]]. The ethics committee of Northwestern Switzerland (EKNZ; 2014_001) approved the trial and it was registered at ClinicalTrials.gov in August 2015 (https://clinicaltrials.gov/ct2/show/NCT02517476).
A total of eight secondary and tertiary care hospitals in Switzerland participated, including the University Clinic in Aarau, the University Hospital in Bern, the Cantonal hospitals in Lucerne, Solothurn, St. Gallen, Muensterlingen and Baselland, and the hospital in Lachen.
2.2. Patient population and randomization
For the trial, we screened medical patients upon hospital admission for risk for malnutrition based on the Nutritional Risk Screening 2002 (NRS 2002) [19]. This nutritional risk score includes different items about the current nutritional status and about severity of the underlying disease [20]. Principal inclusion criteria for the trial were nutritional risk based on a NRS 2002 ≥ 3 points, an expected length of stay of >4 days and written informed consent. We excluded patients initially treated in intensive care or a surgical unit, with inability for oral ingestion of food, with ongoing nutritional support on admission, terminally ill patients, patients with a past medical history of gastric bypass, anorexia nervosa, cystic fibrosis and stem cell transplantation, hospitalized for acute pancreatitis or liver failure, or patients with any contraindications for nutritional support. We randomized patients based on an interactive web-system in a 1:1 ratio to either the intervention group, receiving individual nutritional support to meet their nutritional requirements or the control group receiving standard hospital food.
For this secondary analysis, we only included patients with confirmed lower respiratory tract infections including community-acquired pneumonia, viral pneumonia, exacerbation of chronic obstructive pulmonary disease (COPD) and bronchitis. We collected data on microbiology of patients including blood cultures, PCT tests and urine antigen tests. We used the GOLD classification for staging severity of COPD (Global Initiative for Chronic Obstructive Lung Disease) [21].
2.3. Study intervention
Details of the nutritional intervention, which was in line with current ESPEN guidelines for polymorbid patients [22], have been published previously [16,17,23]. In brief, patients, randomized to the intervention group, received individual nutritional support supervised by a registered dietician. To predict energy goals, the weight-adjusted Harris–Benedict equation was used. A daily protein intake of 1.2–1.5 g/kg body weight was recommended for the general population with lower targets for those with an acute renal failure (0.8 g per kg of body weight). Throughout the hospital stay, the achievement of the individual nutritional plan was reassessed every 24–48 h. If at least 75% of the energy and protein goals could not be reached within 5 days by oral feeding, an escalation of the nutritional support to enteral or even parenteral feeding was made. When discharged, dietary counselling was offered in combination with a prescription for an oral nutritional supplements as needed. Patients in the control group received standard hospital food according to their ability and desire to eat, with no nutritional consultation and no recommendation for additional nutritional support.
2.4. Outcomes
The primary endpoint of this analysis is all-cause mortality up to day 30 after inclusion in the trial. To verify outcome information, trained study nurses performed structured telephone interviews with all patients 30 days after inclusion. If the patient was unable to provide information, a family member or the family doctor confirmed their survival status.
Secondary endpoints included major adverse events, major complications, non-elective hospital readmission within the first 30 days as well as mean length of hospital stay. In line with the initial EFFORT trial [16,17], major adverse events included all-cause mortality, admission to the intensive care unit from the medical ward, non-elective hospital readmission after discharge, and major complications including adjudicated nosocomial infection, respiratory failure, a major cardiovascular event (i.e., stroke, intracranial bleeding, cardiac arrest, myocardial infarction) or pulmonary embolism, acute renal failure, gastro-intestinal events (including hemorrhage, intestinal perforation, acute pancreatitis) or a decline in functional status of 10% or more from admission to day 30 measured by the Barthel's index (scores range from 0 to 100, with higher scores indicating better functional status) [24]. Detailed definitions for each component of the primary endpoint of the main trial were summarized in the original report [16,17].
Protocol adherence was defined by reaching of at least 75% of protein and calorie goals within 5 days of the hospital stay.
2.5. Statistical analyses
We tested the hypothesis that individualized nutritional support was superior to standard hospital food in the subgroup of patients with lower respiratory tract infection. We performed all analyses in the intention-to-treat population, which included all patients with lower respiratory tract infection who had undergone randomization unless they withdrew consent. For the primary outcome, we compared frequencies using a chi-square test. We also fitted a logistic regression model adjusted for main prognostic factors (Barthel's index and NRS 2002 at baseline) and study center as predefined in the study protocol. We reported adjusted odds ratios (OR) and corresponding 95% confidence intervals (CI's). We used a similar statistical approach for secondary endpoints, with use of Student's T test and linear regression models for continuous outcomes. We also used the Kaplan–Meier method to calculate the probability of all-cause mortality within 30 days of randomization. Because the sample size of this subgroup was small and not powered for our primary endpoint, we compared effects to the findings from the main trial by calculation of interaction analyses (test for effect modification). We also performed subgroup analyses based on type of infection and severity of COPD.
We performed all statistical analyses with STATA 15.1 (Stata Corp, College Station, TX, USA). Statistical significance was considered for a P value < 0.05 (for a 2-sided test).
3. Results
3.1. Patient characteristics and protocol adherence
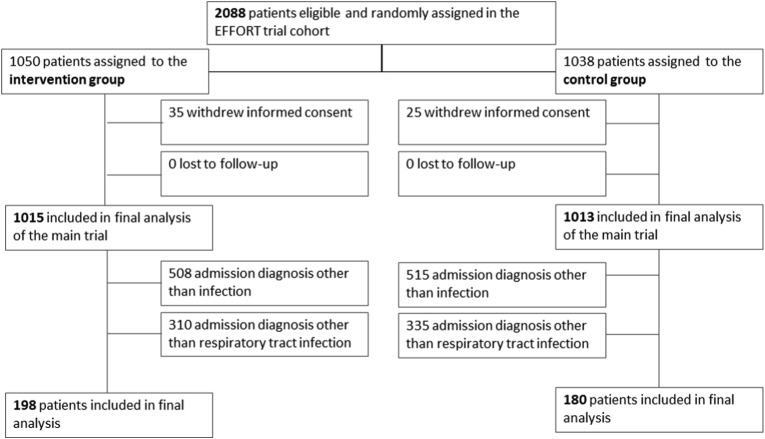
We analyzed data of 378 patients with confirmed lower respiratory tract infection from an initial cohort of 2028 patients (19%). Figure 1 shows the patient flow in the overall trial. Patients had a mean age of 74.4 years and high burden of comorbidities including COPD in 24.1%. Detailed baseline characteristics are presented in Table 1 and main outcomes in Table 2 . Baseline characteristics were well balanced between intervention compared to control group patients. Overall, in 22.8% of patients, the pathogen causing the infection of the lung was isolated (17.5% bacteria [streptococcus pneumonia in 26%], in 4.2% a respiratory virus and in 1.1% a fungus).
Fig. 1.
Patient flow.
Table 1.
Baseline characteristics of all patients with lower respiratory tract infections.
| Parameter | Intervention |
Control |
p value |
|---|---|---|---|
| (n = 198) | (n = 180) | ||
| Sociodemographics | |||
| Age (years), mean (SD) | 73.5 (13.5) | 75.3 (12.7) | 0.17 |
| Different age categories, n (%) | |||
| ≤60 years | 32 (16.2%) | 20 (11.1%) | 0.35 |
| 60–75 years | 66 (33.3%) | 61 (33.9%) | |
| ≥75 years | 100 (50.5%) | 99 (55.0%) | |
| Male gender, n (%) | 109 (55.1%) | 108 (60.0%) | 0.33 |
| Comorbidities, n (%) | |||
| Pre-existing diagnosis of COPD | 41 (20.7%) | 50 (27.8%) | 0.11 |
| GOLD 1 | 2 (9%) | 3 (10%) | 0.85 |
| GOLD 2 | 9 (41%) | 12 (39%) | |
| GOLD 3 | 6 (27%) | 6 (19%) | |
| GOLD 4 | 5 (23%) | 10 (32%) | |
| Coronary heart disease | 72 (36.4%) | 63 (35.0%) | 0.78 |
| Congestive heart failure | 58 (29.3%) | 54 (30.0%) | 0.88 |
| Arterial hypertension | 106 (53.5%) | 93 (51.7%) | 0.72 |
| History of stroke | 14 (7.1%) | 20 (11.1%) | 0.17 |
| Peripheral artery disease | 27 (13.6%) | 26 (14.4%) | 0.82 |
| Chronic kidney disease | 68 (34.3%) | 52 (28.9%) | 0.26 |
| Diabetes mellitus | 45 (22.7%) | 53 (29.4%) | 0.14 |
| Malignancy | 77 (38.9%) | 65 (36.1%) | 0.58 |
| Dementia | 16 (8.1%) | 12 (6.7%) | 0.60 |
| Nutritional assessment | |||
| BMI (kg/m2), mean (SD) | 24.8 (4.8) | 24.9 (5.2) | 0.88 |
| Nutritional risk score 2002, n (%) | |||
| 3 points | 50 (25.3%) | 54 (30.0%) | 0.65 |
| 4 points | 72 (36.4%) | 66 (36.7%) | |
| 5 points | 57 (28.8%) | 47 (26.1%) | |
| >5 points | 19 (9.6%) | 13 (7.2%) | |
| Pathogens responsible for respiratory tract infection, n (%) | |||
| Bacterial pathogen | 33 (16.7%) | 33 (18.3%) | 0.8 |
| Streptococcus pneumonia | 11 (33%) | 11 (33%) | |
| Legionella pneumonia | 4 (9%) | 3 (8%) | |
| Bacteria of gastrointestinal origin | 3 (7%) | 3 (8%) | |
| Mycoplasma pneumonia | 3 (7%) | 1 (2%) | |
| other | 12 (26%) | 15 (38%) | |
| Viral infection | 10 (5.1%) | 6 (3.3%) | 0.91 |
| Influenza | 6 (60%) | 2 (33%) | |
| Metapneumovirus | 1 (10%) | 1 (17%) | |
| Rhinovirus | 3 (30%) | 2 (33%) | |
| Other type of virus | 0 (0%) | 1 (17%) | |
| Fungal infection | 3 (1.5%) | 1 (0.6%) | 0.62 |
| Unknown pathogen | 152 (76.8%) | 140 (77.8%) | |
| Initial blood results | |||
| C-reactive protein (CRP, mg/dl), median (IQR) | 106 (41, 182) | 116.5 (42, 190) | 0.65 |
| White blood count (WBC, x 109 cells per liter), median (IQR) | 8.945 (6.75, 13.43) | 10.24 (7.88, 14.43) | 0.032 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IQR, interquartile range; SD, standard deviation.
p-values are derived from chi-square tests for categorical variables, Student's T-test for normal distributed and Mann–Whitney tests for non-normal distributed (i.e. CRP, WBC) variables.
Table 2.
Summary of binary outcomes of patients with respiratory tract infection compared to the overall medical trial (EFFORT) population.
| Parameters | Intervention group (n = 198) | Control group (n = 180) | Odds ratio (OR), Subgroup analysis (95% CI and p-value) |
Odds ratio (OR), EFFORT cohort (95% CI and p-value) | p Interaction |
|---|---|---|---|---|---|
| Primary endpoint: mortality at 30 days | |||||
| All-cause mortality, n (%) | 18 (9.1%) | 22 (12.2%) | 0.47 (0.17, 1.27); p = 0.136 | 0.65 (0.47, 0.91), p = 0.011 | 0.859 |
| Secondary outcomes | |||||
| Adverse outcomesa within 30 days n (%) | 42 (21.2%) | 48 (26.7%) | 0.64 (0.28, 1.44); p = 0.278 | 0.79 (0.64, 0.97), p = 0.023 | 0.993 |
| Any major complicationsb within 30 days, n (%) | 20 (10.1%) | 15 (8.3%) | 1.32 (0.45, 3.86); p = 0.61 | 0.95 (0.68, 1.34), p = 0.788 | 0.332 |
| Non-elective hospital readmission within 30 days, n (%) | 9 (4.5%) | 12 (6.7%) | 0.37 (0.03, 4.48); p = 0.435 | 0.99 (0.73, 1.35), p = 0.958 | 0.293 |
| Functional outcomes | |||||
| Decline in functional status> 10% at 30 daysc, n (%) | 21 (10.6%) | 26 (14.4%) | 0.49 (0.2, 1.23); p = 0.129 | 0.62 (0.4, 0.96), p = 0.034 | 0.908 |
SD, standard deviation; OR, odds ratio.
Composite endpoint consisting of all-cause mortality at 30 days, major complications within 30 days, admission to the intensive care unit from the medical ward and non-elective readmissions after discharge.
Composite endpoint consisting of adjudicated nosocomial infection, respiratory failure, a major cardiovascular event (i.e., stroke, intracranial bleeding, cardiac arrest, myocardial infarction) or pulmonary embolism, acute renal failure, gastro-intestinal events (including hemorrhage, intestinal perforation, acute pancreatitis).
To estimate decline in functional status, we used Barthel index (score ranging from 0 to 100, with higher scores indicating better functional status) and compared scores at admission with scores at 30 days. Only surviving patients were included in this analysis.
Protocol adherence was high and energy and protein goals were met in 79% and 76% in the intervention group respectively. Energy and protein intake in the intervention group was significantly higher compared to control group patients (mean difference in daily energy intake of 286 kcal (95% CI 226 to 541) and in mean daily protein intake of 13 g (95%CI 6 to 20). The same was true in regard to weight adjusted individual targets with significantly higher intakes of calories (adjusted mean difference of 4.1 kcal/kg/day, [95%CI 3.3 to 4.9] and protein (adjusted mean difference of 0.14 g/kg/day [95%CI 0.11 to 0.17]) in intervention group compared to control group patients. Table 3 gives detailed results regarding nutritional outcomes within the trial.
Table 3.
Summary of continuous outcomes of patients with respiratory tract infection compared to the overall medical trial (EFFORT) population.
| Parameters | Intervention group (n = 198) | Control group (n = 180) | Adjusted difference, Subgroup analysis (95% CI and p-value) |
Adjusted difference, EFFORT cohort (95% CI and p-value) | p Interaction |
|---|---|---|---|---|---|
| Hospital outcomes | |||||
| length of stay (days), median (IQR) | 7.0 (5.0, 13.0) | 8.0 (6.0, 12.0) | 1.65 (−0.38, 3.68); p = 0.111 | −0.21 (−0.76, 0.35), p = 0.46 | 0.072 |
| Nutritional intake during first 10 days of hospital stay | |||||
| Caloric intake (kcal/day), mean (SD) | 1426 (569) | 1140 (478) | 383 (226, 541); p < 0.001 | 290 (240, 340), p < 0.001 | 0.223 |
| Weight adjusted caloric intake (kcal/kg/day), mean (SD) | 22.5 (8.9) | 17.6 (7.4) | 4.5 (2.9, 6.2); p < 0.001 | 4.1 (3.3, 4.9), p < 0.001 | 0.380 |
| Protein intake (g/day), mean (SD) | 56.0 (21.4) | 41.6 (16.1) | 13 (7, 20); p < 0.001 | 10 (8, 12), p < 0.001 | 0.441 |
| Weight adjusted protein intake (g/kg/day), mean (SD) | 0.84 (0.35) | 0.68 (0.29) | 0.15 (0.08, 0.21); p < 0.001 | 0.14 (0.11, 0.17), p < 0.001 | 0.492 |
| Functional outcomes | |||||
| Barthel score (points) at day 30, mean (SD) | 95.0 (9.0) | 95.8 (9.1) | −1.63 (−4.85, 1.6); p = 0.32 | 3.26 (0.93, 5.6), p = 0.006 | 0.185 |
IQR, interquartile range; SD, standard deviation; OR, odds ratio.
3.2. Clinical outcomes
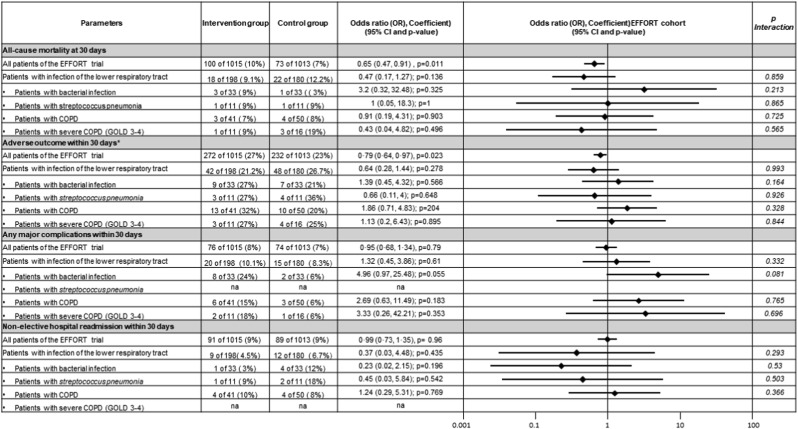
Lower respiratory tract infection patients receiving nutritional support had a 25%-reduction in the risk of 30-day mortality (22/180 [12.2%] vs 18/198 [9.1%]), which was per se not significant in this subgroup (odds ratio 0.47 [95%CI 0.17 to 1.27]) but similar to the significant mortality benefits observed in the overall EFFORT cohort (OR 0.65, 95% CI 0.47 to 0.91). There was no evidence for a subgroup effect (p for interaction = 0.859). This result was also robust among the predefined subgroup analyses stratified by evidence of bacterial infection vs no bacterial infection, streptococcus pneumonia infection vs. other types of infection, underlying COPD vs no COPD and underlying severe COPD (GOLD stages 3–4) vs less severe or no COPD (Fig. 2 ), with again no significant results in the interaction analysis.
Fig. 2.
Odds ratios for primary and secondary outcomes in pre-specified subgroups (Forest plot). Abbreviations: COPD = chronic obstructive pulmonary disease, GOLD = Global Initiative for Chronic Obstructive Lung Disease.
There were also similar improvements as compared to the main trial regarding secondary outcomes including major adverse outcome (48/198 (26.7%) vs 42/180 (21.2%), OR 0.64 (95%CI 0.28 to 1.44); p for interaction = 0.993) and functional outcome expressed by a decline of the Barthel index >10% at 30 days (26 (14.4%) vs 21 (10.06%), OR 4.49 (95%CI 0.2° to 1.23); p for interaction = 0.629). Table 2 (for binary outcomes), Table 3 (for linear outcomes) and Fig. 2 show results for the different endpoints in patients with lower respiratory tract infections as compared to the overall EFFORT trial and also present results from interaction analysis investigating whether patients with lower respiratory tract infections show a different response to nutritional treatment compared to the overall EFFORT population.
4. Discussion
Within this secondary analysis of a large randomized clinical trial, we investigated effects of individualized nutritional support on mortality and other important clinical outcomes in the subgroup of patients with lower respiratory tract infection. We found a 25%-reduction in 30-day mortality and also strong reductions in the risk of adverse outcome associated with nutritional support in patients with lower respiratory tract infections at nutritional risk, which was similar to the general medical inpatient population of the main trial (EFFORT).
Overall, the evidence that nutritional support reduces mortality, complications and length of hospital stay in patients with bacterial or viral infections remains weak due to lack of larger scale trials specifically focusing on this population of patients. Also, in our report, we only had a relatively small group of patients with respiratory tract infections importantly limiting the power of the analysis and results in the subgroup regarding mortality, complications and other outcomes were not significant per se. However, We also compared our subgroup analysis to the results of the main trial for evidence of a subgroup effect, i.e., whether there was evidence that patients with respiratory infection would behave differently from other rmedical patients in regard to the nutritional support intervention. Importantly, we did not find that patients with respiratory infections had a different response to nutritional support compared to the overall medical population. Therefore, we believe that the positive effects of nutritional support on mortality and other secondary outcomes may also be true for patients with respiratory tract infection at nutritional risk – including COVID-19 patients. Clearly, our data needs validation in an independent and well powered sample to draw firm conclusions. Such a trial should then also investigate whether there are differences in response to nutritional treatment among patients with severe systemic infections vs those with more local infections, among different types of pathogens and among patients infected with Sars-CoV 2 (Severe acute respiratory syndrome coronavirus 2) compared to other pathogens.
Despite the lack of evidence regarding nutritional support in non-critically ill patients with infections, there have been several reports in the general medical patient population showing that nutritional support in at risk patients has positive effects on outcomes [3,[25], [26], [27]]. Effects were stronger if subjects were malnourished at admission to the hospital compared to patients classified as at risk for malnutrition [28]. Importantly, while most studies on the effect of early nutritional support by oral, enteral order parenteral route on clinical outcome were performed either in the intensive care setting, surgical collectives or patients presenting with acute pancreatitis [29], there are several trials for patients on the medical ward showing a reduced risk of infectious and non-infectious complications in treated patients [2,[30], [31], [32], [33]]. To our knowledge, however, there is no study specifically looking at patients with lower respiratory tract infection [[30], [31], [32],[34], [35], [36], [37]]. Herein, we believe our data provide novel and timely information.
Since the publication of the EPaNIC trial, concerns have been raised about potential harmful effects of early and high caloric nutritional support among intensive care patients with sepsis [38,39]. Casaer et al. found worse clinical outcomes with increased infectious complications and prolonged length of hospital and intensive care stay in critically-ill patients treated with parenteral nutrition in addition to partial enteral nutrition [38]. Yet, the subsequent EDEN trial comparing a full enteral feeding strategy with a strategy of initial trophic enteral feeding for up to 6 days in critically-ill patients with an acute lung injury did not observe worse clinical outcomes [40]. Also, results did not differ in the one-year follow-up of the EDEN cohort [41]. The EPaNIC trial, however, included a different patient population in regard to older age, burden of comorbidities and prevalence of malnutrition [42]. Unlike in critical care, there is no data suggesting potential harmful effects of overfeeding on normal medical wards – although high inflammation has been shown to be an effect modifier on the association of nutritional support and outcome [43]. Still, our data showing improved outcomes of an individualized nutritional approach for this highly vulnerable population of patients with respiratory infection is reassuring. Importantly, our approach was based mainly on optimizing oral intake using hospital kitchen food with the addition of oral nutritional supplements. Additional EN or PN was used only in a low number of patients if after 5 days nutritional goals could not be reached. We thus had little risk of overfeeding of patients [44].
In our trial, we did not use full-replacement feeding in intervention group patients, but rather aimed to reach at least 75% of protein (and calorie) goals over 5 days (1.2–1.5 g protein/kg/day) by use of an individualized nutritional support algorithm according to a previously published feeding protocol with individual definition of each patient's nutritional goals [23]. As a result the mean protein intake during the hospital stay in intervention group patients was (only) 0.84 g protein/kg/day, which was much higher compared to control group patients not receiving nutritional support (mean of 0.68 g protein/kg/day), but still not in the optimal range as outlined in the nutritional protocol. As patients typically showed a stepwise increase in nutritional intake over the course of the hospital stay, a majority of intervention group patients eventually reached their individual protein (and calorie) goals by day 5 by means of oral nutrition with no need to escalate to enteral or parenteral routes. Importantly, it is possible that higher intakes of proteins (and calories) during the initial hospital stay would have further beneficial effects on patient outcomes, but this would require a separate trial with higher specific protein and calorie goals. It is possible that for such a trial the use of enteral and/or parenteral routes may be necessary in higher proportions of patients to reach higher goals.
Some researchers have suggested that specific formulae, generally referred to as immuno-nutrition including high amounts of arginine, glutamine, branched chain amino acids, n-3 fatty acids, and nucleotides, may provide further benefit for infection patients also including COVID-19 patients. Yet, such treatments were not studied in the EFFORT trial, nor to our knowledge, in any other trial [[45], [46], [47]]. Still, some data suggest that a diet enriched with eicosapentaenoic acid (EPA) and g-linolenic acid (GLA) may favorably reduce the pulmonary inflammatory response and support vasodilation and oxygenation in septic patients [48]. These benefits observed in pre-clinical studies, however, did not result in a clinical benefit according to a recent Cochrane review focusing on critically-ill patients with acute respiratory distress syndrome (ARDS) [49]. There is at least one prospective randomized controlled trial planned looking specifically at the role of immune-nutrition for the COVID-19 patient population (https://clinicaltrials.gov/ct2/show/NCT04249050).
5. Conclusion
This subgroup analysis from a large nutrition support trial suggests that patients presenting to the hospital with bronchopulmonary infection and nutritional risk as assessed by NRS 2002 may show a mortality benefit from individualized inhospital nutritional support. The small sample size and limited statistical power calls for larger nutritional studies focusing on this highly vulnerable patient population.
Author contribution
Prof. Philipp Schuetz was the principal investigator of this trial and was responsible for obtaining funding, drafting the trial protocol, data analysis and interpretation, and writing of the final report.
Annic Baumgartner, Flavia Hasenboehler, Jennifer Cantone, Lara Hersberger, Annika Bargetzi, Laura Bargetzi, Nina Kaegi-Braun and Pascal Tribolet were involved in drafting the protocol, data collection and approved the final version of the manuscript.
Filomena Gomes, Claus Hoess, Vojtech Pavlicek, Stefan Bilz, Sarah Sigrist, Michael Brändle, Christoph Henzen, Robert Thomann, Jonas Rutishauser, Drahomir Aujesky, Nicolas Rodondi and Jacques Donzé were involved in drafting the initial trial protocol, supervision of study sites, drafting of the final manuscript and approved the final version of the manuscript.
Zeno Stanga and Beat Mueller were involved in obtaining funding, drafting the trial protocol, supervision of study sites, drafting of the final manuscript and approved the final version of the manuscript.
Conflict of interest
The study was investigator-initiated and supported by a grant from the Swiss National Science Foundation to P.Schuetz (SNSF Professorship, PP00P3_150531) and the Forschungsrat of the Kantonsspital Aarau (1410.000.058 and 1410.000.044). The institution of P.Schuetz has previously received unrestricted grant money unrelated to this project from Neste Health Science and Abbott Nutrition. The institution of Z.Stanga received speaking honoraria and research support from Neste Health Science, Abbott Nutrition and Fresenius Kabi. All other authors report no conflicts of interest.
Acknowledgments
We thank all patients and hospital staff for support of the EFFORT trial.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2020.10.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Schuetz P., Greenwald J.L. Web exclusive. Annals for hospitalists inpatient notes - optimizing inpatient nutrition-why hospitalists should get involved. Ann Intern Med. 2020;172 doi: 10.7326/M20-0120. HO2-HO3. [DOI] [PubMed] [Google Scholar]
- 2.Blum C.A., Schuetz P., Nigro N., Winzeler B., Arici B., Refardt J., et al. Blackwell Publishing Ltd; 2019. Cosyntropin testing does not predict response to glucocorticoids in community-acquired pneumonia in a randomized controlled trial. Clinical Endocrinology. [DOI] [PubMed] [Google Scholar]
- 3.Felder S., Braun N., Stanga Z., Kulkarni P., Faessler L., Kutz A., et al. Unraveling the link between malnutrition and adverse clinical outcomes: association of acute and chronic malnutrition measures with blood biomarkers from different pathophysiological states. Ann Nutr Metabol. 2016;68:164–172. doi: 10.1159/000444096. [DOI] [PubMed] [Google Scholar]
- 4.Felder S., Lechtenboehmer C., Bally M., Fehr R., Deiss M., Faessler L., et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition. 2015;31:1385–1393. doi: 10.1016/j.nut.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Imoberdorf R., Meier R., Krebs P., Hangartner P.J., Hess B., Staubli M., et al. Prevalence of undernutrition on admission to Swiss hospitals. Clin Nutr. 2010;29:38–41. doi: 10.1016/j.clnu.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Yeo H.J., Byun K.S., Han J., Kim J.H., Lee S.E., Yoon S.H., et al. Prognostic significance of malnutrition for long-term mortality in community-acquired pneumonia: a propensity score matched analysis. Korean J Intern Med. 2019;34:841–849. doi: 10.3904/kjim.2018.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L., et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74:871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puig-Domingo M., Marazuela M., Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu G., Zhang S., Mao Z., Wang W., Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr. 2020;74:876–883. doi: 10.1038/s41430-020-0659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caccialanza R., Laviano A., Lobascio F., Montagna E., Bruno R., Ludovisi S., et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74:110835. doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12 doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaegi-Braun N., Baumgartner A., Gomes F., Stanga Z., Deutz N.E., Schuetz P. Evidence-based medical nutrition - a difficult journey, but worth the effort! Clin Nutr. 2020 Oct;39(10):3014–3018. doi: 10.1016/j.clnu.2020.01.023. Epub 2020 Feb 5. PMID: 32061370. [DOI] [PubMed] [Google Scholar]
- 14.Gomes F., Baumgartner A., Bounoure L., Bally M., Deutz N.E., Greenwald J.L., et al. Association of nutritional support with clinical outcomes among medical inpatients who are malnourished or at nutritional risk: an updated systematic review and meta-analysis. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bally M.R., Blaser Yildirim P.Z., Bounoure L., Gloy V.L., Mueller B., Briel M., et al. Nutritional support and outcomes in malnourished medical inpatients: a systematic review and meta-analysis. JAMA Intern Med. 2016;176:43–53. doi: 10.1001/jamainternmed.2015.6587. [DOI] [PubMed] [Google Scholar]
- 16.Schuetz P., Fehr R., Baechli V., Geiser M., Deiss M., Gomes F., et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393:2312–2321. doi: 10.1016/S0140-6736(18)32776-4. [DOI] [PubMed] [Google Scholar]
- 17.Schuetz P., Fehr R., Baechli V., Geiser M., Gomes F., Kutz A., et al. Design and rationale of the effect of early nutritional therapy on frailty, functional outcomes and recovery of malnourished medical inpatients trial (EFFORT): a pragmatic, multicenter, randomized-controlled trial. Int J Clin Trials. 2018;5:142–150. [Google Scholar]
- 18.Kaegi-Braun N., Tribolet P., Gomes F., Fehr R., Baechli V., Geiser M., et al. Six-month outcomes after individualized nutritional support during the hospital stay in medical patients at nutritional risk: secondary analysis of a prospective randomized trial. Clin Nutr. 2021 Mar;40(3):812–819. doi: 10.1016/j.clnu.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Kondrup J., Rasmussen H.H., Hamberg O., Stanga Z., Ad Hoc E.W.G. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 20.Hersberger L., Bargetzi L., Bargetzi A., Tribolet P., Fehr R., Baechli V., et al. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: secondary analysis of a prospective randomised trial. Clin Nutr. 2020;39:2720–2729. doi: 10.1016/j.clnu.2019.11.041. [DOI] [PubMed] [Google Scholar]
- 21.Global initiative for chronic obstructive lung disease - pocket Guide to COPD. 2020. [Google Scholar]
- 22.Gomes F., Schuetz P., Bounoure L., Austin P., Ballesteros-Pomar M., Cederholm T., et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37:336–353. doi: 10.1016/j.clnu.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Bounoure L., Gomes F., Stanga Z., Keller U., Meier R., Ballmer P., et al. Detection and treatment of medical inpatients with or at-risk of malnutrition: suggested procedures based on validated guidelines. Nutrition. 2016;32:790–798. doi: 10.1016/j.nut.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 25.Sobotka L. Basics in Clinical Nutrition: nutritional support in different clinical situations. e-SPEN. 2010 [Google Scholar]
- 26.Cederholm T., Bosaeus I., Barazzoni R., Bauer J., Van Gossum A., Klek S., et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr. 2015;34:335–340. doi: 10.1016/j.clnu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Deutz N.E., Matheson E.M., Matarese L.E., Luo M., Baggs G.E., Nelson J.L., et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr. 2016;35:18–26. doi: 10.1016/j.clnu.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Rypkema G., Adang E., Dicke H., Naber T., de Swart B., Disselhorst L., et al. Cost-effectiveness of an interdisciplinary intervention in geriatric inpatients to prevent malnutrition. J Nutr Health Aging. 2004;8:122–127. [PubMed] [Google Scholar]
- 29.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 30.Pupelis G., Selga G., Austrums E., Kaminski A. Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition. 2001;17:91–94. doi: 10.1016/s0899-9007(00)00508-6. [DOI] [PubMed] [Google Scholar]
- 31.Malhotra A., Mathur A.K., Gupta S. Early enteral nutrition after surgical treatment of gut perforations: a prospective randomised study. J Postgrad Med. 2004;50:102–106. [PubMed] [Google Scholar]
- 32.Kaur N., Gupta M.K., Minocha V.R. Early enteral feeding by nasoenteric tubes in patients with perforation peritonitis. World J Surg. 2005;29:1023–1027. doi: 10.1007/s00268-005-7491-z. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 33.Barlow R., Price P., Reid T.D., Hunt S., Clark G.W., Havard T.J., et al. Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clin Nutr. 2011;30:560–566. doi: 10.1016/j.clnu.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Bozzetti F., Braga M., Gianotti L., Gavazzi C., Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001;358:1487–1492. doi: 10.1016/S0140-6736(01)06578-3. [DOI] [PubMed] [Google Scholar]
- 35.Gupta R., Patel K., Calder P.C., Yaqoob P., Primrose J.N., Johnson C.D. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (Apache II > or =6) Pancreatology : Off J Int Assoc Pancreat. 2003;3:406–413. doi: 10.1159/000073657. [DOI] [PubMed] [Google Scholar]
- 36.Eckerwall G.E., Axelsson J.B., Andersson R.G. Early nasogastric feeding in predicted severe acute pancreatitis: a clinical, randomized study. Ann Surg. 2006;244:959–965. doi: 10.1097/01.sla.0000246866.01930.58. discussion 65-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrov M.S., Kukosh M.V., Emelyanov N.V. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23:336–344. doi: 10.1159/000097949. discussion 44-5. [DOI] [PubMed] [Google Scholar]
- 38.Casaer M.P., Mesotten D., Hermans G., Wouters P.J., Schetz M., Meyfroidt G., et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 39.Bertolini G., Iapichino G., Radrizzani D., Facchini R., Simini B., Bruzzone P., et al. Early enteral immunonutrition in patients with severe sepsis: results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med. 2003;29:834–840. doi: 10.1007/s00134-003-1711-5. [DOI] [PubMed] [Google Scholar]
- 40.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Rice T.W., Wheeler A.P., Thompson B.T., Steingrub J., Hite R.D., et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012 Feb 22;307(8):795–803. doi: 10.1001/jama.2012.137. Epub 2012 Feb 5. PMID: 22307571; PMCID: PMC3743415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Needham D.M., Dinglas V.D., Bienvenu O.J., Colantuoni E., Wozniak A.W., Rice T.W., et al. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. Br Med J. 2013;346:f1532. doi: 10.1136/bmj.f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phan K.A., Dux C.M., Osland E.J., Reade M.C. Effect of hypocaloric normoprotein or trophic feeding versus target full enteral feeding on patient outcomes in critically ill adults: a systematic review. Anaesth Intensive Care. 2017;45:663–675. doi: 10.1177/0310057X1704500604. [DOI] [PubMed] [Google Scholar]
- 43.Merker M., Felder M., Gueissaz L., Bolliger R., Tribolet P., Kagi-Braun N., et al. Association of baseline inflammation with effectiveness of nutritional support among patients with disease-related malnutrition: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schutz P., Bally M., Stanga Z., Keller U. Loss of appetite in acutely ill medical inpatients: physiological response or therapeutic target? Swiss Med Wkly. 2014;144:w13957. doi: 10.4414/smw.2014.13957. [DOI] [PubMed] [Google Scholar]
- 45.Baumgartner A., Kagi-Braun N., Tribolet P., Gomes F., Stanga Z., Schuetz P. Individualised nutritional support in medical inpatients - a practical guideline. Swiss Med Wkly. 2020;150:w20204. doi: 10.4414/smw.2020.20204. [DOI] [PubMed] [Google Scholar]
- 46.Aeberhard C., Mayer C., Meyer S., Mueller S.A., Schuetz P., Stanga Z., et al. Effect of preoperative immunonutrition on postoperative short-term outcomes of patients with head and neck squamous cell carcinoma. Head Neck. 2018;40:1057–1067. doi: 10.1002/hed.25072. [DOI] [PubMed] [Google Scholar]
- 47.Mueller S.A., Mayer C., Bojaxhiu B., Aeberhard C., Schuetz P., Stanga Z., et al. Effect of preoperative immunonutrition on complications after salvage surgery in head and neck cancer. J Otolaryngol Head Neck Surg. 2019;48:25. doi: 10.1186/s40463-019-0345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pontes-Arruda A., Martins L.F., de Lima S.M., Isola A.M., Toledo D., Rezende E., et al. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in the early treatment of sepsis: results from a multicenter, prospective, randomized, double-blinded, controlled study: the INTERSEPT study. Crit Care. 2011;15 doi: 10.1186/cc10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dushianthan A., Cusack R., Burgess V.A., Grocott M.P., Calder P.C. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst Rev. 2019;1 doi: 10.1002/14651858.CD012041.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.