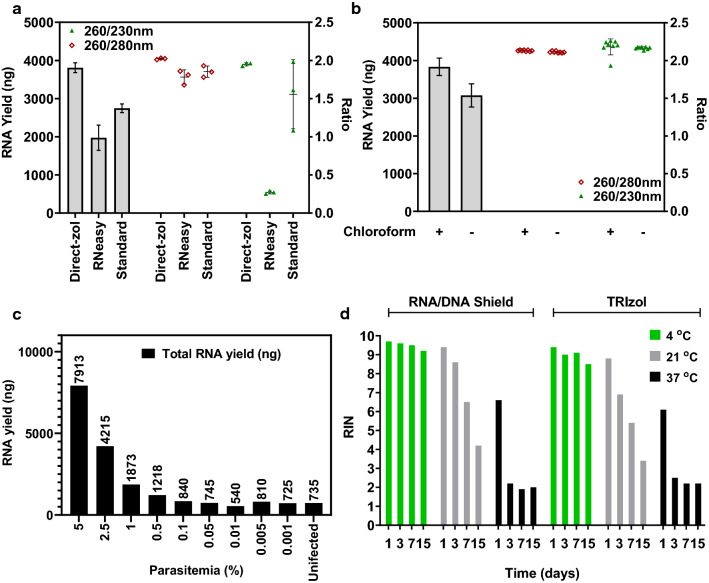
Fig. 1.
Optimization of RNA extraction and sample preservation methods for P. falciparum infected blood samples. a Comparison of RNA extraction methods. Total RNA yields, obtained from 500 µl of packed iRBCs (12 hpi, ring stage, 2% parasitaemia) are shown in grey bars. RNA purity assessed with Nanodrop at 230, 260 and 280 nm wavelengths shown as ratios. b Effect of chloroform supplementation on total yield and purity of RNA extracted from 500 µl of packed iRBCs (12 hpi, ring stage, 2% parasitaemia) using Direct-zol extraction method. Chloroform presence or absence is indicated below the graph as + or—respectively. RNA purity assessed with Nanodrop at 230, 260 and 280 nm wavelengths shown as ratios. c Expected total RNA yields obtained from 500 µl of packed iRBCs infected with a range of parasitaemia (12 hpi, ring stage) obtained by serial dilution of initial parasitaemia of 5%. RNA was extracted from 500 µl of uninfected RBCs as a negative control. d Efficacy of TRIzolTM reagent and DNA/RNA ShieldTM in preservation of RNA extracted from iRBC (20 hpi, young trophozoites, 8% parasitaemia) stored in either TRIzolTM or DNA/RNA ShieldTM were subjected to different temperature conditions for varying periods of time. RNA from samples was extracted using Direct-zol method and analysed on Agilent Bioanalyzer. RIN values were obtained for each condition and are shown here