Figure 1. Trial profile and tumor responses (pathologic and radiologic) to neoadjuvant pembrolizumab.
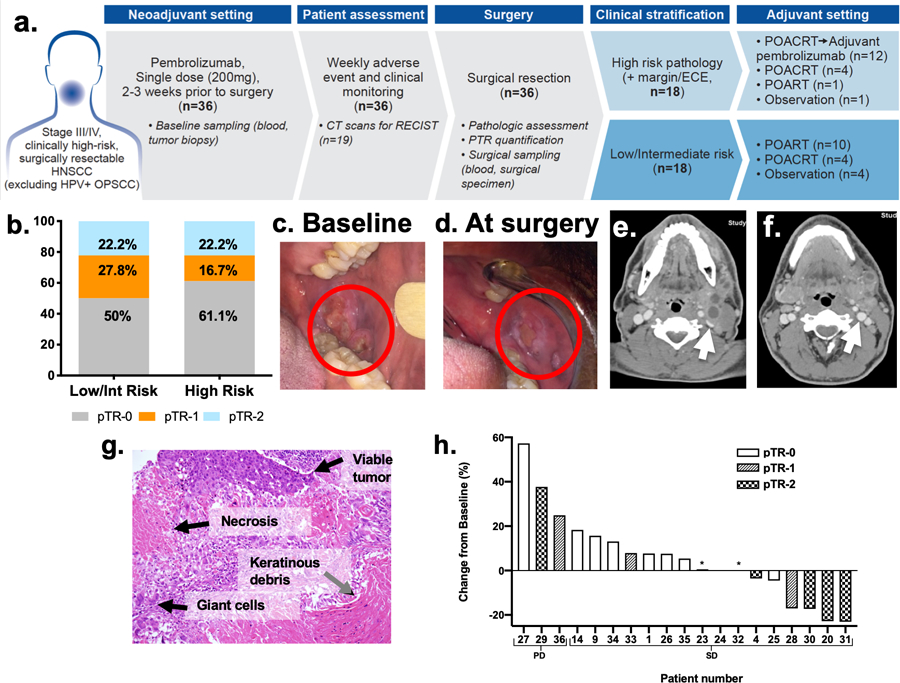
(A) Trial profile: Patients (n=36) with locally advanced, Stage III/IV, HPV-negative HNSCCs underwent baseline tumor and blood sampling and received neoadjuvant pembrolizumab 2–3 weeks before surgery. Of the 18 patients with high-risk pathology, 12 received adjuvant pembrolizumab. Patients with low/intermediate-risk pathology did not receive adjuvant pembrolizumab. (B) pathologic tumor response-2 (pTR-2) was observed in similar proportions of patients with low/intermediate and high-risk pathology. (C) Baseline and (D) post-neoadjuvant treatment (at surgery) images of oral cavity primary cancer in Patient 20 showing dramatic decrease in the size. (E) Representative CT images at baseline and (F) after neoadjuvant pembrolizumab (day prior to surgery), confirmed tumor response seen on physical exam. Notably, the pre-treatment CT and FDG-PET/CT scan (not shown) showed multiple large and necrotic FDG-avid neck lymph nodes, which are radiologic signs of SCC. Of note, the internal jugular vein (white arrow) was compressed on the baseline scan (E) and appeared fuller on the post-treatment scan (F). (G) Representative H&E slide of pTR highlighting changes noted in surgical specimens. (H) Nineteen patients had CT evaluations at baseline and prior to surgery following neoadjuvant pembrolizumab: 16 with stable disease (SD) and 3 with progressive disease (PD) by RECIST criteria. *Indicates two patients with pTR-1.
