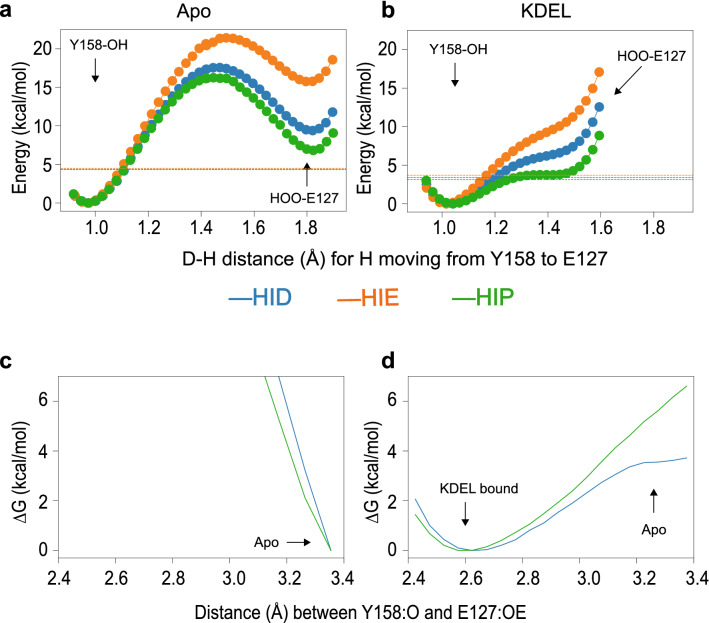
Figure 2.
QM estimate of energy of proton transfer from the Y158 to E127. (a) In the Apo state, a very large barrier (~ 20 kcal/mol) is observed during the proton transfer, which is not observed in the KDEL-bound state. (b) In the KDEL-bound state, the energy difference for the proton movement is smaller than the ZPE (dashed line) when the histidine is protonated (green). The energy difference is greatly increased when histidine is deprotonated (orange and blue). (c,d) QM/MM based energy landscape of separating the hydrogen bond. c. In the Apo state, the short hydrogen bond is prohibited by the larger energy barrier. (d) In the KDEL-bound state, 6.5 kcal/mol of energy is required to break the hydrogen bond when histidine is protonated (green line), while only 3.5 kcal/mol is required when histidine is deprotonated (blue line).