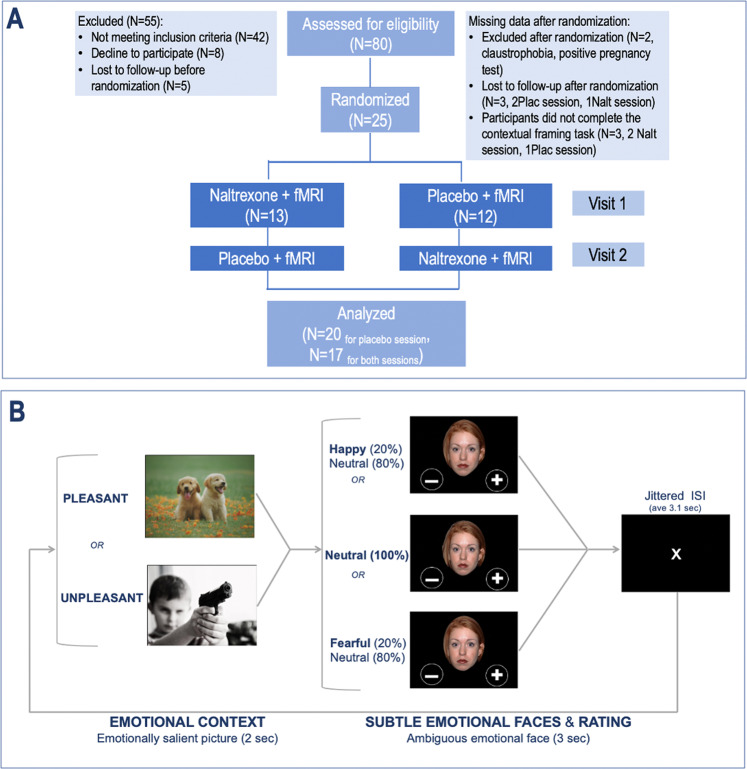
Fig. 1. Study design: participants completed a randomized, double-blind, placebo-controlled, crossover study of one dose of naltrexone 50 mg, or matching placebo.
a, b Contextual framing fMRI task: Participants were first presented with an emotionally salient contextual image (from IAPS) for 2 s (context phase), followed by an ambiguous face to be rated as either positive or negative in 3 s (emotion and rating phase). Note: the examples of contextual images were replaced with similar copyright-free images from the website in order to comply with the IAPS usage agreement.