FIGURE 1.
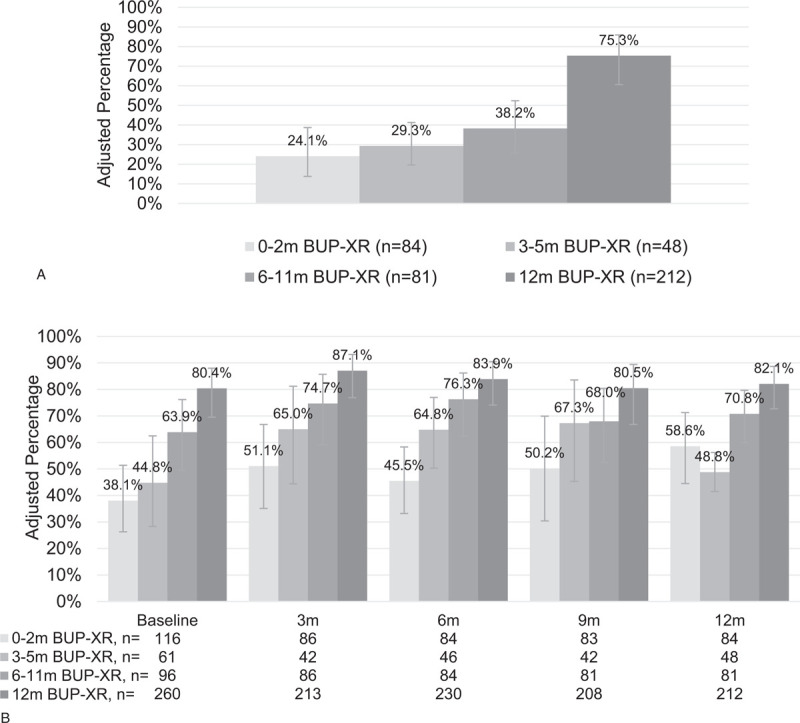
Predicted abstinence over time by within-trial BUP-XR treatment duration.a (A) Sustained self-reported abstinence for 12 months. (B) Self-reported past-week abstinence. BUP-XR, buprenorphine extended-release subcutaneous injections; m, months. aPredicted results from multiple regression results weighted for participant characteristics at the screening visit of their first clinical trial, including self-reported abstinence and controlling for demographics at baseline, illicit substance use history, treatment prior to trial, time from last randomized efficacy or open-label safety BUP-XR injection to RECOVER baseline, and select indicators from public health surveillance data systems.
