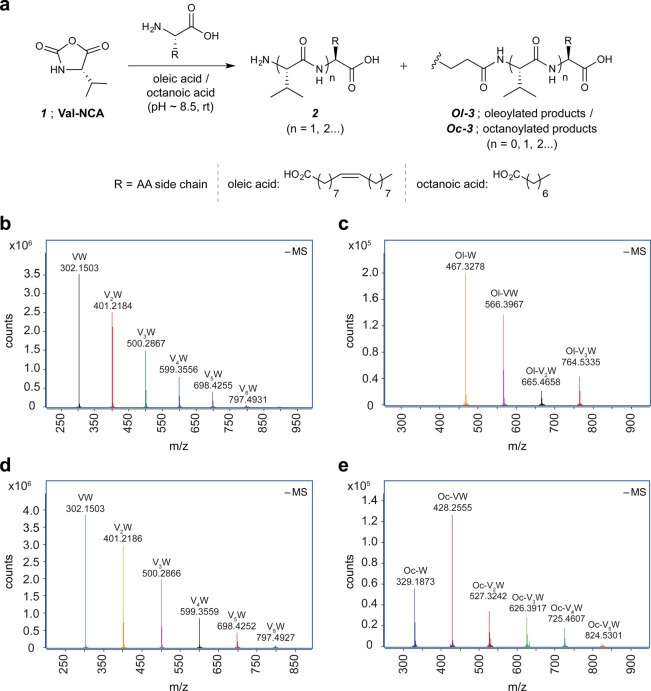
Figure 1.
Val-NCA-mediated acylation of amino acids and peptides. (a) Schematic of Val-NCA-mediated peptide acylation in the presence of either oleic acid or octanoic acid. (b–e) Overlay of extracted high-resolution mass spectrometry (HRMS) spectra ([M – H]−1) for peptide or acyl peptide products observed in 24 h incubated reaction mixtures starting from Val-NCA (20 mM), tryptophan (W) (20 mM), EPPS (300 mM), and either oleic acid (20 mM) (b and c) or octanoic acid (20 mM) (d and e). For similar HRMS analyses of the oligo-valine products (Vn) [R2 = CH(CH3)2], see Figure S1, SI). HRMS experiments were carried out in negative mode. Panels (b) and (d) highlight the W-containing native peptides observed in the presence of oleic acid and octanoic acid, respectively. Panels (c) and (e) highlight the W-containing oleoylated (Ol-) and octanoylated (Oc-) products, respectively. See the SI for tabulation of mass errors and an HPLC-extracted ion chromatogram showing the product distribution observed in the case of oleic acid (b, c).