Abstract
Background: Autologous myoblasts have been tested in the treatment of muscle-related diseases. However, the standardization of manufacturing myoblasts is still not established. Here we report a flask and animal-free medium-based method of manufacturing clinical-grade myoblast together with establishing releasing criteria for myoblast products under Good Manufacturing Practice (GMP). Methods: Quadriceps muscle biopsy samples were donated from three patients with myogenic ptosis. After biopsy samples were digested through enzymatic dissociation, the cells were grown in T175 flasks (passage 0) and hyperflasks (passage 1) in the animal-free SkGMTM-2 skeletal muscle cell growth medium containing 5% human platelet lysate for 15–17 days. The harvested cells were released based on cell morphology, cell dose, viability, sterility, endotoxin, mycoplasma and immunophenotype. Myotube differentiation was also evaluated. Results: 400 to 500 million myoblast cells were manufactured within 15 to 17 days by the end of passage 1, which met pre-determined releasing criteria. The manufactured myoblast cells could differentiate and fuse into myotubes in vitro, with the possible trend that the donor age may impact the differentiation ability of myoblasts. Conclusions: The present study establishes a flask-based method of manufacturing myoblast in the animal-free medium together with releasing criteria, which is simple, robust, inexpensive and easily reproducible. This study will serve as the validation for a planned phase 1 clinical trial to assess the use of autologous myoblast transplants for the treatment of myogenic ptosis and other myogenic diseases.
Electronic supplementary material
The online version of this article (10.1007/s10616-020-00420-9) contains supplementary material, which is available to authorized users.
Keywords: Myoblast, Cell manufacturing, Myogenic ptosis
Introduction
The skeletal muscles make up 40–50% of the body mass. Aging decreases regenerative capacity after injury, leading to a progressive reduction in muscle mass and longer immobilization. In addition, genetic muscle-wasting disorders result in a loss of voluntary movement, compromised quality of life, and premature death. Cell therapy represents a means of counteracting the muscle degeneration and muscle wasting (Blau and Daley 2019; Negroni et al. 2015). Myoblasts are derived from satellite cells, a progenitor cell population located under the basal lamina of skeletal muscle. Upon exercise, muscle injury, or degeneration, satellite cells are activated and become myoblasts, which are further proliferated, differentiated, and fused into multinuclear myotubes and further myofibers (Endo 2015). With the potential regeneration ability, autologous myoblast transplantation has been tested in the treatment of Duchenne muscular dystrophy, ischemic and nonischemic cardiomyopathy, fecal incontinence, and other myogenic degeneration diseases, which demonstrated the safety of autologous myoblast transplantation. This cellular therapy may induce promising clinical responses in some patients (Boyer et al. 2018; Menasche et al. 2008; Miyagawa et al. 2017; Perie et al. 2014).
Given the physical properties of myoblasts (large size and adherence), manufacturing clinically relevant large doses of myoblasts can be difficult if using conventional methods, as this may require many culture flasks and many open procedures. Another variable to consider during the myoblast expansion is the protein source used in the culture, such as fetal bovine serum, human platelet lysate and serum-free media. Different culture media can contribute to the variability of myoblast growth and differentiation (Jarocha et al. 2014). Although several groups have tried different methods to manufacture myoblasts, standardization of manufacturing myoblasts and releasing criteria have not been well established. One group manufactured myoblasts in the modified MCDB120 medium supplemented with bovine fetal serum (Perie et al. 2014), another group manufactured myoblasts in the skBM medium supplemented with bovine serum (Miyagawa et al. 2017). As the use of fetal bovine serum may induce immunologic reactions in recipients, it is better to use animal-free medium or serum-free medium to manufacture cellular products. To this end, we describe our current method of generating myoblasts from human quadriceps muscle in the animal-free culturing medium under GMP condition.
Myogenic ptosis is caused by congenital dysgenesis of the levator muscle or acquired muscular diseases and results in a drooping of the upper eyelid. Conventional ptosis surgery using levator muscle advancement is usually effective for non-myogenic causes of ptosis but is ineffective in the treatment of myogenic ptosis due to levator muscle weakness. A more effective form of treatment for myogenic ptosis is needed. We postulate that the injection of autologous expanded myoblasts into a dystrophic levator muscle might result in muscle tissue regeneration, improved levator muscle function and ptosis improvement. The present study will serve as an initial validation study of myoblast expansion for myogenic ptosis and other potential muscular diseases.
Materials and methods
The study was approved by the University of Manitoba Research Ethics Board. All donors with myogenic ptosis provided informed consent for study enrolment. All cell culture manipulations, quality and release testing, and flow cytometry were performed at the Manitoba Centre for Advanced Cell and Tissue Therapy (MCACTT) in Winnipeg, Manitoba, Canada, which is accredited by Foundation for the Accreditation of Cellular Therapy (FACT) for more than minimal manipulation. The quadriceps muscle biopsy procedure was performed at the Misericordia Buhler Eye Center Procedure room using local anesthetic under aseptic technique. The reagents and supplies used in this study are listed in the Supplementary Table 1.
To be included in this validation, individuals had to be age 18 or over and have myogenic ptosis. Patients were excluded if they were pregnant, had an active infection, active inflammatory disease, active cancer, were undergoing chemotherapy, or unable to provide informed consent. Similar exclusion and inclusion criteria will be used in a planned phase 1 clinical trial to assess the use of autologous myoblast transplants for the treatment of myogenic ptosis.
Quadriceps muscle biopsy procedure
In the procedure room, the donor was placed in a supine position, and the skin over the biopsy site was cleansed with povidone iodine. A sterile drape was applied over the thigh. Local anesthetic (~ 10 ml of 2% lidocaine) was injected into the biopsy area. Using a #15 blade, a 5 cm longitudinal incision was made over the vastus lateralis muscle at the mid to upper thigh level. Hemostasis was obtained using bipolar cautery. The incision was spread apart using blunt dissection with scissors to expose the vastus lateralis. The muscle was clamped with hemostats proximally and distally. A segment of muscle measuring approximately 4 cm × 1 cm was excised with a #15 blade. The excised muscle was immediately placed in a sterile container with pre-chilled sterile normal saline. The clamped portions of the muscle were cauterized prior to release of the clamps. A 4-0 polyglactin 910 suture was used for fascial closure and layered skin closure.
Muscle tissue digestion
For all donors, 3 to 5 grams of muscle tissue were placed in pre-chilled saline and immediately transferred to the GMP facility. A minimum volume of 20 mL of tissue digestion solution Collagenase-Dispase-CaCl2 was prepared as described in the Supplementary Table 2 and warmed to 37 °C before tissue dissociation. The tissue was placed in a sterile petri dish with 3 ml of 37 °C warmed tissue digestion solution. Fibrotic and fatty tissues were discarded as much as possible, and within 10 min, the muscle was torn quickly and gently into small but distinguishable pieces (approximately > 0.5 mm2) with sterile Covidien Curity Suture Removal Kit Littauer Scissors and Metal forceps. The sample was then incubated in 10 ml digestion solution at 37 °C for up to 30 to 40 min. If fragments of muscle remained, a second incubation was performed on the remaining pieces and with fresh enzymatic solution for another 30 to 40 min. Three to four volumes of Dulbecco’s Modified Eagle Medium (DMEM) with 5% human platelet lysate (PLTMax) were used to stop tissue digestion, and then the muscle solution was filtered through a 100 µm cell strainer. The muscle solution was centrifuged to collect the pellet and followed by pellet resuspension into myoblast complete expansion media (SkGMTM-2 skeletal muscle cell growth medium without fetal bovine serum, containing 5% human platelet lysate) prepared as described in the Supplementary Table 2.
Cell seeding
The cell suspension was equally distributed into 3 to 4 Cellbind T175 flasks within 30 ml myoblast complete expansion media and incubated for 90 min in a humidified 37 °C, 5% CO2 incubator. This step allowed the contaminating fibroblasts to adhere but not the myoblasts. The spending medium containing the non-adherent cells was collected and transferred into another 3 to 4 new T175 flasks for further incubation.
Media change and cell observation
The first media change was performed 4 to 5 days after seeding. The spending media was aspirated, and the flasks were washed with Dulbecco’s phosphate-buffered saline (DPBS) twice. A total of 30 ml fresh myoblast complete expansion media was added into each flask for further incubation. The myoblasts were fed with fresh medium every 3 to 4 days. The cell morphology, confluence, and contamination were observed under microscope.
Myoblast passage
When groups of growing loci were visible and the cell confluence was around 30% to 50% under the microscope, they were passaged into hyperflasks, usually performed 10 to 11 days after initial seeding. The cells were lifted by TrypLE Select. After counting the cells, 500 to 1,000 cells/cm2 were seeded into 3–4 hyperflasks and cultured in myoblast complete expansion media. The myoblasts were fed with fresh medium at Day 3–4 post passaging.
Myoblast harvesting
Cells were harvested when cell confluence reached 70% to 80%. Media were aspirated from the hyperflasks, and the hyperflasks were washed with DPBS once. The cells were then lifted by TrypLE Select. The cell suspension was centrifuged to collect the cell pellet and resuspended in PlasmaLyte A with 5% human albumin. The sample was aliquoted for cell counting, viability, phenotypic analysis, sterility, endotoxin, mycoplasma, and myotube in vitro forming assay.
Myoblast cryopreservation
Before cryopreservation, the myoblasts were centrifuged at 450 g for 10 min and washed with a pre-chilled rinsing buffer containing PlasmaLyte A with 20% human albumin. The cells were resuspended in pre-chilled rinsing buffer at half of the final freezing volume. After placing the cells in a refrigerator for 20 to 30 min, 2× cryopreservation buffer containing 20% dimethyl sulfoxide (DMSO), 20% human albumin, and 60% PlasmaLyte A was added. The cells were cryopreserved using a control-rate freezer according to our standard freezing protocol for hematopoietic stem cell products (SOP CTL010 Cryopreservation). Briefly, in controlled rate freezing, the concentrated myoblasts were frozen down at a rate of 1 °C/min up to a temperature point of −60 °C; then, the freezing process down to a target of −90 °C was performed at 3 °C/min. Once the product temperature reached − 90 °C, the product was transferred into the vapor phase of liquid nitrogen tank for storage.
Flow cytometry
The viability by Annexin V and 7-AAD staining and immunophenotype of myoblasts were evaluated by flow cytometry techniques established in our facility (Guan et al. 2017; Guan et al. 2018). To evaluate the immunophenotype of myoblasts, 0.5 × 106 myoblasts were washed in DPBS with 2% fetal bovine serum, and then incubated with 20 μl purified human FcR binding inhibitor for 10 min. This was followed by staining with fluorescence APC-labeled antibodies against CD45 for 20 min according to the manufacturer recommendations. To evaluate the intracellular expression of MyoD, the cells were fixed and permeabilized using eBioscience intracellular fixation/permeabilization buffer, and then stained with APC-labeled antibodies against MyoD for 20 min. After staining, cells were acquired and analyzed using flow cytometry (FACS CantoII, BD Bioscience).
Myotube forming assay
To evaluate whether myoblast can differentiate and infuse into myotubes, myoblasts were seeded into a 6-well plate and cultured and differentiated in the MyoCult™ Differentiation Medium according to the protocols provided by StemCell Technologies. After culturing for 7 to 10 days, the cells were stained the myosin heavy chain to evaluate myotube formation.
Immunocytochemical staining
The cells were first fixed with fixation buffer for 20 to 30 min at room temperature, permeabilized with permeabilization solution (Mix 50 μl of Triton X-100 with 10 ml of 1x DPBS) for 3 to 4 min, and then blocked with blocking buffer (Mix together 1 ml of fetal bovine serum, 10 μl of Triton X-100, and 9 ml of 1x DPBS) for 30 min, and further stained with fluorescence labeled antibodies at the concentration 1:100 for overnight at 4 °C, including Alex Fluor 488–labeled myosin 4 monoclonal antibody (MF20), APC-labeled anti-MyoD, PE labeled antibodies against Myf5 and myogenin, efluor 660 labeled anti-Desmin, or isotype control antibodies. The cells were mounted with Prolong gold antifade reagent with DAPI after washing and then visualized under a fluorescent microscope. Fusion index is calculated as percentage nuclei in myosin heavy chain (MF20) positive myotubes of total nuclei.
Releasing tests
The manufactured myoblast cell products were evaluated and released based on the pre-determined releasing criteria listed in Table 1.
Table 1.
Releasing Criteria for Myoblast Product
| Test | Method | Acceptance criteria |
|---|---|---|
| Cell morphology | Examination of morphology of adherent cells by light microscopy prior to preparation of final cell product | ≥ 50% of culture surface area covered by attached cells with the presence of bipolar, elongated, and spindle shape cells |
| Signs of contamination prior to final cell product preparation | Microscopic inspection | Absence of visible evidence of contamination |
| Viability | 7-AAD/annexin V/trypan blue | ≥ 80% cell viability of cells |
| Endotoxin | Endosafe LAL assay | < 5EU/kg patient weight |
| Mycoplasma | MycoAlert | Negative |
| Sterility | BACT/Alert (aerobic and anaerobic culture) | Negative |
| Cell count | Manual and automatic cell count | 1–2 x 106/kg |
| Myoblast phenotype | Flow cytometry |
% MyoD expression ≥ 50% % CD45 expression < 5% |
EU, endotoxin units; MyoD, myoblast determination protein 1; 7-AAD, 7-aminoactinomycin D
Results
All donors had satisfactory healing of the quadriceps muscle biopsy site. There were no instances of infection, hematoma formation, or wound dehiscence, and all donors were able to resume normal activities within 1 month from biopsy.
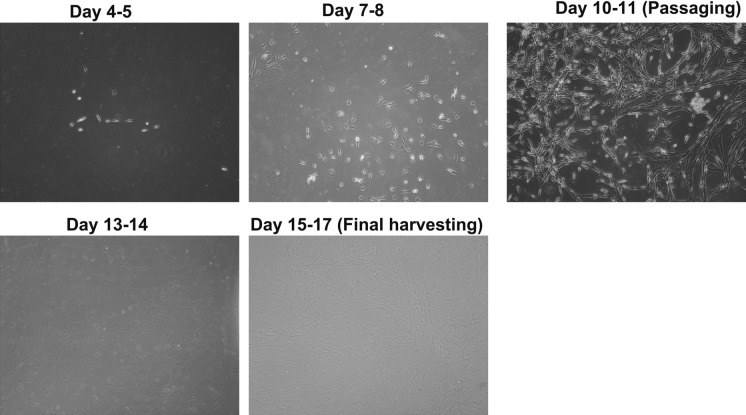
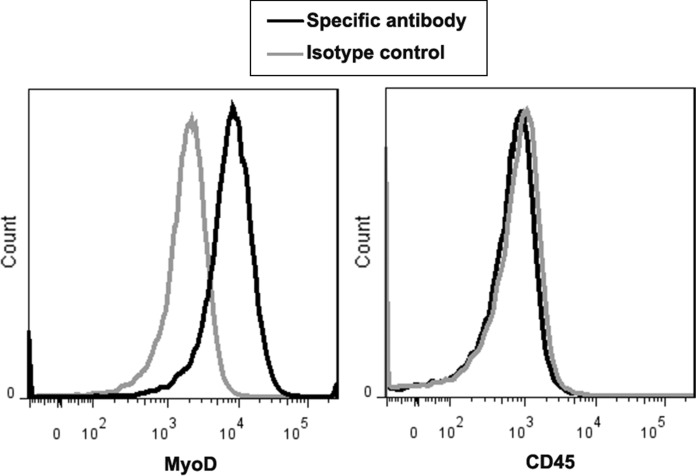
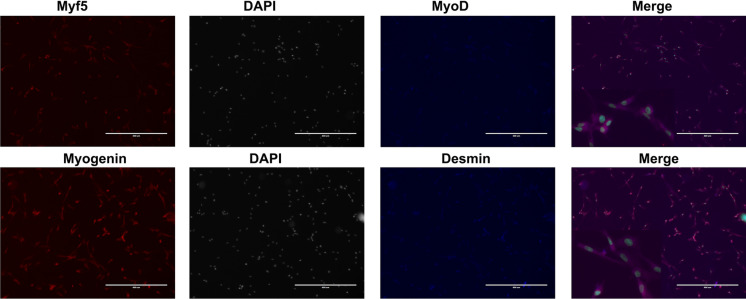
Using our flask-based method, large numbers of myoblasts were successfully manufactured with elongated and spindle shape (Fig. 1) within 15 to 17 days by the end of passage 1 with the average cell yield 509.5 x 106; this was enough for 2 to 3 transplantation cell doses (1–2 × 106/kg/dose) (Table 2). The manufactured cells had good viability (> 87%) and expressed the myogenic specific transcription factor MyoD (≥ 65%), and this was negative of CD45 by flow cytometry (Fig. 2). To confirm the expression of MyoD and evaluate whether the manufactured myoblasts also expressed other muscle regulatory factors (Myf5 and myogenin) and cytoskeletal protein Desmin, immunocytochemical staining analysis was performed on the cells. Greater than 90% of these cells expressed MyoD (Fig. 3). They also expressed Myf5, Myogenin, and Desmin, which indicated that the majority of manufactured cells were primary committed myoblasts (MyoD+Myf5+Myogenin+Desmin+)(Bentzinger et al. 2012; Endo 2015).
Fig. 1.
Morphology of myoblast expanded from skeletal muscle of donors on different culturing days. The morphology was evaluated under light microscope and taken pictures on different culturing days
Table 2.
Summary of manufacturing myoblasts
| Donors | Donor 1 | Donor 2 | Donor 3 |
|---|---|---|---|
| Donor age (years) | 79 | 51 | 58 |
| Gender | M | M | M |
| Culturing days from seeding muscle cells to passaging | 10 | 10 | 11 |
| Cell number of P0 (× 106) | 28.8 | 11.5 | 15.4 |
| Culturing days from passaging to final harvest | 7 | 5 | 6 |
| Final cell yield (× 106) | 565.8 | 458.8 | 504 |
| Cell viability (%) (acceptance criteria: > 80%) | |||
| Trypan blue | 95.4% | 94.7% | 96.3% |
| 7-AAD | 87% | 98.5% | 96.9% |
| Annexin V | 92.1% | 97.6% | 98.4% |
| Phenotype by flow cytometry (%) | |||
| MyoD | 75.0% | 68.5% | 65% |
| CD45 | 0.6% | 0.3% | 0.1% |
| Phenotype by immunocytochemical staining (%) | |||
| Myf5 | >90% | >90% | >90% |
| Desmin | >90% | >90% | >90% |
| Myogenin | >90% | >90% | >90% |
| Sterility test of final product (aerobic and anaerobic) | Negative | Negative | Negative |
| Endotoxin | < 1EU/kg | < 1 EU/kg | < 1 EU/kg |
| Mycoplasma testing | Negative | Negative | Negative |
| In vitro myotube formation | Very few myotubes observed; fusion index < 1% | Large number of myotubes observed; fusion index 22.3% | A number of myotubes observed; fusion index 5.4% |
EU, endotoxin units; MyoD, myoblast determination protein 1; 7-AAD, 7-aminoactinomycin D
Fig. 2.
The immunophenotype of myoblast expanded from skeletal muscles of donors. To evaluate the expression of MyoD and CD45 on myoblast (passage 1), fluorescent-labeled antibodies against MyoD and CD45 were used to stain the myoblast, and then the cell staining was analyzed by flow cytometry. Representative expression of MyoD and CD45 on myoblast expanded from one donor
Fig. 3.
Immunocytochemical staining analysis of Desmin, Myf5, MyoD, and myogenin of the myoblast. To evaluate the expression of muscle regulatory factors (Myf5, MyoD, and myogenin) and the cytoskeletal protein Desmin on the manufactured myoblast products, myoblast was stained with fluorescence-labeled antibodies against Myf5, MyoD, myogenin, and Desmin. The cells were mounted with Prolong gold antifade reagent with DAPI and visualized under a fluorescent microscope. Representative expression of Myf5, MyoD, myogenin, and Desmin on myoblast expanded from donors. Scale bar size 400μm
There was no evidence of microbial contamination, as demonstrated by negative aerobic/anaerobic cultures, endotoxin, and Mycoplasma tests in all samples. Taken together, the manufactured myoblasts met our pre-determined releasing criteria (Table 1).
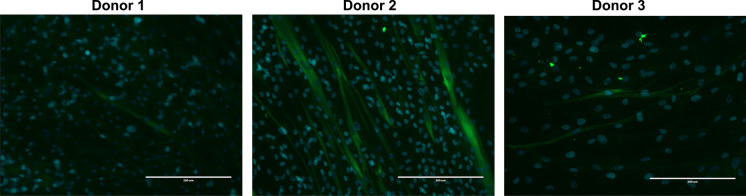
We further evaluated whether the ex vivo expanded myoblasts can differentiate and fuse into myotubes. We showed that the expanded myoblasts are able to form myotubes; however, donor age may impact the differentiation ability (Fig. 4). The fusion index of the younger (age 51) donor-derived ex vivo expanded myoblasts was 22.3%, and the fusion index of the older (age 79) donor-derived ex vivo expanded myoblasts was less than 1% (Table 2).
Fig. 4.
In vitro differentiation of myoblast. To evaluate whether the manufactured myoblast product can differentiate into myotube in vitro, myoblast cells were culture in the MyoCult™ Differentiation Medium according to the protocols provided by StemCell Technologies for 7–10 days. Then the cells were stained with Alex Fluor 488 labeled antibodies (MF20) against the myosin heavy chain to evaluate myotube formation. The cells were mounted with Prolong gold antifade reagent with DAPI and visualized under a fluorescent microscope. Scale bar size 200μm
Discussion
Transplantation of autologous ex vivo expanded myoblasts has been tested in the treatment of multiple muscular-related diseases, such as fecal incontinence, dysphagia, and cardiomyopathy (Boyer et al. 2018; Miyagawa et al. 2017; Perie et al. 2014). However, the methods of myoblast expansion for clinical application have not been standardized. Prior studies varied in their methods to expand myoblasts as well as the culture medium and protein sources used (Miyagawa et al. 2017; Perie et al. 2014). Animal derived serum was widely used in the expansion of autologous myoblast in the majority of reported clinical applications(Boyer et al. 2018; Menasche et al. 2008; Miyagawa et al. 2017; Perie et al. 2014; Peters et al. 2014; Sawa et al. 2015), which has the disadvantage of potentially inducing immunological reactions post infusion. In the present study, by working on muscle biopsy specimens donated from patients with myogenic ptosis, we described a flask-based method to manufacture myoblasts in a commercial culture medium with animal-free serum.
All donors in our study demonstrated excellent myoblast cell yield and cell viability. The safety profile of the myoblasts was shown by negative microbial cultures, negative endotoxin, and negative Mycoplasma tests in all samples. By using flow cytometry method, it showed over 65% myoblast cells expressed muscle regulatory factor MyoD. Further, the expression of muscle regulatory factors Myf5, MyoD, and myogenin, and cytoskeletal protein Desmin in myoblast cells was examined by immunofluoresence staining with fluorescence labeled primary antibodies. Although the staining was not strong and the background was not as clear as the indirect immunofluorescence labeling, the present data showed that the manufactured myoblast cells expressed Myf5, MyoD, myogenin and Desmin. In the future, indirect immunofluorescence labeling will be used for examining the expression of these proteins. Taken together, this indicated these cells were mainly committed myoblasts. Studies have shown that after muscle injury, the muscle satellite cells/progenitor cells will be activated and proliferated to be committed myoblasts (Myf5+MyoD+Myogenin+), which will further differentiate into myocytes and myotubes/myofibers (Bentzinger et al. 2012; Endo 2015).
Previous studies have shown varying results regarding the influence of donor age on the growth of myoblast in vitro. Bonavaud et al. showed that there was a trend toward decreasing myoblast growth with increasing donor age but this was not statistically significant (Bonavaud et al. 1997). Another study showed that myoblasts from older donors (age 74 to 76 years) grew much slower than those from younger donors (age 4 months to 16 years), and took much longer to fuse into myotubes (Corbu et al. 2010). On the other hand, some reports showed donor age did not significantly influence the cell growth and/or myogenicity (Baj et al. 2005; Price et al. 2014). In the present study, we showed that myoblasts isolated from three donors had similar cell growth and cell yield; however, the youngest donor (Donor 2, age 51) had higher myotube formation (fusion index 22.3%) within 10 days of differentiation medium culture in vitro when compared to the other two donors. Our findings indicated a trend towards decreasing propensity for myotube formation with increasing donor age.
One limitation of this study is that the cell number post muscle tissue digestion before seeding into T175 flasks, was not counted. Based on literature review, approximately 107 cells might be obtained from per gram skeletal muscle tissue (Spinazzola and Gussoni 2017). In the present study, approximately 3–5 g muscle tissue derived cells were equally seeded into 3–4 T175 flasks (expecting 1–1.5 × 107/flask). In the future, cell number will be counted before seeding into T175 flasks.
In conclusion, our study shows that quadriceps muscle-derived myoblasts can be manufactured in a GMP facility that meets the pre-determined releasing criteria based on cell morphology, cell dose, viability, sterility, endotoxin, mycoplasma and immunophenotype. The flask-based method of manufacturing myoblast presented in this study is simple, robust, inexpensive and easily reproducible; enough transplantation doses of primary myoblasts can be manufactured within 3 weeks by the end of passage 1. This study serves as a validation for a future phase 1 clinical trial to assess the use of autologous myoblast transplants for the treatment of myogenic ptosis, in which the thawed myoblast will be placed in the culture for 1 day to recover from the freezing process prior to injection into the patient. The protocol of manufacturing myoblasts and the releasing tests described herein can also be potentially applicable for the use of myoblasts to treat other muscle-related diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by Misericordia Health Centre Foundation.
Authors’ contributions
MLW, DS and QG conceived and designed the project; MLW recruited donors; QG developed the cell manufacturing protocol; AL, MLW and AF performed muscle biopsy; KA, MT, QG and AG manufactured cell products; QG analyzed the data; MLW, DS and QG wrote the manuscript; AL, AF, KA, MT and AG critically reviewed it. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Ethics approval and consent to participate
The study was approved by the University of Manitoba Research Ethics Board. Animal study was not used in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baj A, Bettaccini AA, Casalone R, Sala A, Cherubino P, Toniolo AQ. Culture of skeletal myoblasts from human donors aged over 40 years: dynamics of cell growth and expression of differentiation markers. J Transl Med. 2005;3:21. doi: 10.1186/1479-5876-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger, C.F., Wang, Y.X., and Rudnicki, M.A. (2012). Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol 4 [DOI] [PMC free article] [PubMed]
- Blau HM, Daley GQ. Stem Cells in the Treatment of Disease. N Engl J Med. 2019;380:1748–1760. doi: 10.1056/NEJMra1716145. [DOI] [PubMed] [Google Scholar]
- Bonavaud S, Thibert P, Gherardi RK, Barlovatz-Meimon G. Primary human muscle satellite cell culture: variations of cell yield, proliferation and differentiation rates according to age and sex of donors, site of muscle biopsy, and delay before processing. Biol Cell. 1997;89:233–240. doi: 10.1111/j.1768-322X.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Boyer O, Bridoux V, Giverne C, Bisson A, Koning E, Leroi AM, Chambon P, Dehayes J, Le Corre S, Jacquot S, et al. Autologous Myoblasts for the Treatment of Fecal Incontinence: results of a Phase 2 Randomized Placebo-controlled Study (MIAS) Ann Surg. 2018;267:443–450. doi: 10.1097/SLA.0000000000002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbu A, Scaramozza A, Badiali-DeGiorgi L, Tarantino L, Papa V, Rinaldi R, D’Alessandro R, Zavatta M, Laus M, Lattanzi G, et al. Satellite cell characterization from aging human muscle. Neurol Res. 2010;32:63–72. doi: 10.1179/174313209X385725. [DOI] [PubMed] [Google Scholar]
- Endo T. Molecular mechanisms of skeletal muscle development, regeneration, and osteogenic conversion. Bone. 2015;80:2–13. doi: 10.1016/j.bone.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Guan Q, Ezzati P, Spicer V, Krokhin O, Wall D, Wilkins JA. Interferon gamma induced compositional changes in human bone marrow derived mesenchymal stem/stromal cells. Clin Proteomics. 2017;14:26. doi: 10.1186/s12014-017-9161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Q, Li Y, Shpiruk T, Bhagwat S, Wall DA. Inducible indoleamine 2,3-dioxygenase 1 and programmed death ligand 1 expression as the potency marker for mesenchymal stromal cells. Cytotherapy. 2018;20:639–649. doi: 10.1016/j.jcyt.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Jarocha D, Stangel-Wojcikiewicz K, Basta A, Majka M. Efficient myoblast expansion for regenerative medicine use. Int J Mol Med. 2014;34:83–91. doi: 10.3892/ijmm.2014.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- Miyagawa, S., Domae, K., Yoshikawa, Y., Fukushima, S., Nakamura, T., Saito, A., Sakata, Y., Hamada, S., Toda, K., Pak, K., et al. (2017). Phase I Clinical Trial of Autologous Stem Cell-Sheet Transplantation Therapy for Treating Cardiomyopathy. J Am Heart Assoc 6 [DOI] [PMC free article] [PubMed]
- Negroni E, Gidaro T, Bigot A, Butler-Browne GS, Mouly V, Trollet C. Invited review: stem cells and muscle diseases: advances in cell therapy strategies. Neuropathol Appl Neurobiol. 2015;41:270–287. doi: 10.1111/nan.12198. [DOI] [PubMed] [Google Scholar]
- Perie S, Trollet C, Mouly V, Vanneaux V, Mamchaoui K, Bouazza B, Marolleau JP, Laforet P, Chapon F, Eymard B, et al. Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: a phase I/IIa clinical study. Mol Ther. 2014;22:219–225. doi: 10.1038/mt.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KM, Dmochowski RR, Carr LK, Robert M, Kaufman MR, Sirls LT, Herschorn S, Birch C, Kultgen PL, Chancellor MB. Autologous muscle derived cells for treatment of stress urinary incontinence in women. J Urol. 2014;192:469–476. doi: 10.1016/j.juro.2014.02.047. [DOI] [PubMed] [Google Scholar]
- Price DM, Lane FL, Craig JB, Nistor G, Motakef S, Pham QA, Keirstead H. The effect of age and medical comorbidities on in vitro myoblast expansion in women with and without pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2014;20:281–286. doi: 10.1097/SPV.0000000000000064. [DOI] [PubMed] [Google Scholar]
- Sawa Y, Yoshikawa Y, Toda K, Fukushima S, Yamazaki K, Ono M, Sakata Y, Hagiwara N, Kinugawa K, Miyagawa S. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J. 2015;79:991–999. doi: 10.1253/circj.CJ-15-0243. [DOI] [PubMed] [Google Scholar]
- Spinazzola, J.M., and Gussoni, E. (2017). Isolation of Primary Human Skeletal Muscle Cells. Bio Protoc 7 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.