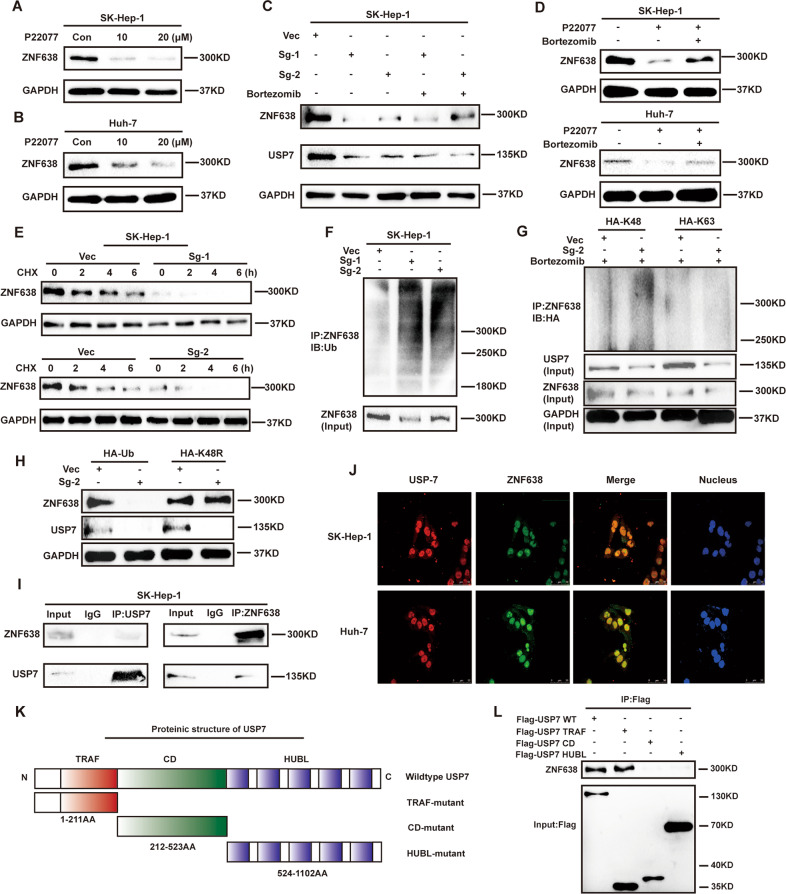
Fig. 1. USP7 stabilizes ZNF638 protein partially through its deubiquitinating activity.
A, B ZNF638 protein level was down-regulated with the treatment of P22077 in SK-Hep1 and Huh-7 cells. C The knockdown efficiency of USP7 by two specific SgRNA (Sg1, Sg2) and their effects on ZNF638 expression were determined by immunoblotting in SK-Hep1 cells; proteasome inhibitor Bortezomib (100 nM 8 h) partially rescued declined expression of ZNF638 in USP7-deficient cells. D P22077 (20 μM 24 h) induced ZNF638 protein inhibition was partially rescued by Bortezomib (100 nM 8 h) in SK-Hep1 and Huh-7 cells. E The half-life of ZNF638 protein in normal and USP7-deficient SK-Hep1 cells was determined by using CHX (100 μg/ml) at indicated time points. F Genetic inhibition of USP7 in SK-Hep1 cells increased ubiquitination level of ZNF638. G The enhanced polyubiquitination of ZNF638 in SK-Hep1 cells according to USP7 knockdown was mainly Lys48 but not Lys 63 linked polyubiquitination. H K48-resistant ubiquitin (Lys48 to Arg48) could reverse ZNF638 expression in USP7-deficient SK-Hep1 cells. I ZNF638 and USP7 were endogenously interacted with each other in SK-Hep1 cells as determined by Co-IP assay. J USP7 and ZNF638 were mainly co-localized in cell nucleus as determined by immunofluorescence in Huh-7 and SK-Hep1 cells. K Schematic illustration of the protein structure of USP7. L Three of flag tagged truncated mutant plasmids (Flag-TRAF, Flag-CD, and Flag-HUBL) and wild-type USP7 (Flag-USP7) were transfected into SK-Hep1 cells, followed by immunoprecipitation using flag antibody and immunoblotting with ZNF638 antibody. Each immunoblotting assay was performed at least three times from independent studies.