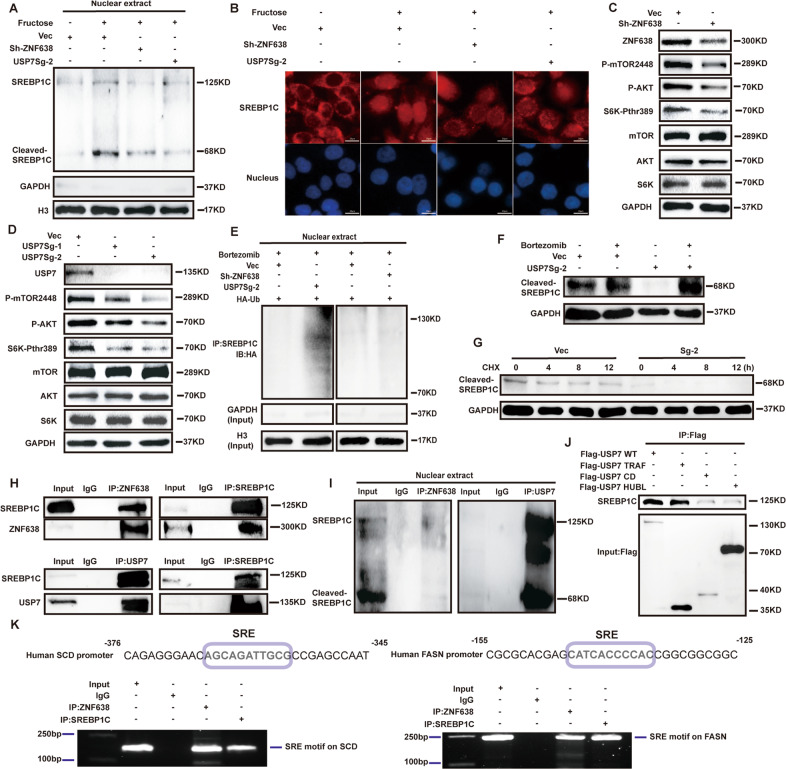
Fig. 5. USP7 and ZNF638 control the nuclear accumulation of cleaved-SREBP1C.
A The effects of USP7 or ZNF638 knockdown on fructose (20 mM 48 h) induced cleaved-SREBP1C were verified by immunoblotting, using nuclear extracts from SK-Hep1 cells. B The subcellular location changes of SREBP1C upon the fructose induction (20 mM 48 h) with or without USP7, ZNF638 knockdown were explored by immunofluorescence in SK-Hep1 cells. C Genetic ablation of ZNF638 in SK-Hep1 cells reduced phosphorylation levels of mTOR, AKT, and S6K. D Genetic ablation of USP7 in SK-Hep1 cells reduced phosphorylation levels of mTOR, AKT, and S6K. E USP7 but not ZNF638 knockdown could increase the ubiquitination of cleaved-SREBP1C in SK-Hep1 cells. F The decreased cleaved-SREBP1C resulting from USP7 knockdown could be rescued by bortezomib (100 nM 8 h) in SK-Hep1 cells. G The half-life of cleaved-SREBP1C in USP7-deficient SK-Hep1 cells was shortened. H The reciprocal interactions between ZNF638/USP7 and full-length SREBP1C were observed by Co-IP assays in SK-Hep1 cells. I The endogenous interactions between ZNF638/USP7 and cleaved-SREBP1C were determined by Co-IP assays using nuclear lysates of SK-Hep1 cells. J The binding sites of USP7 on SREBP1C were determined by transfecting truncated USP7 mutants (Flag-TRAF, Flag-CD, and Flag-HUBL) into SK-Hep1 cells, following immunoprecipitation with flag antibody and detection of SREBP1C. K The binding of ZNF638 on the promoters of SREBP1C target genes were examined via ChIP assay in SK-Hep1 cells, lanes of IgG and SREBP1C were set as negative and positive control.