Figure 2:
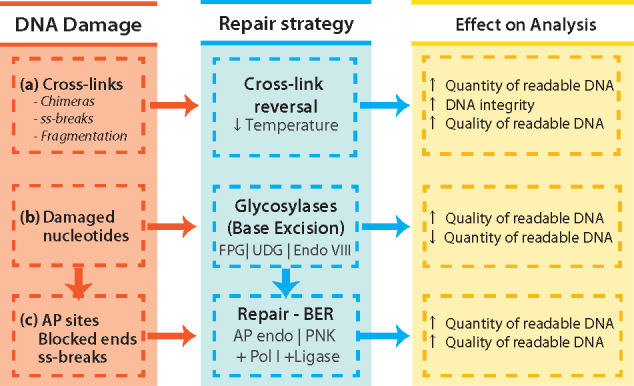
Summary of strategies applied for improving integrity, quantity and quality of bacterial DNA derived from FFPE specimens. (a) Exposure of DNA to denaturing temperatures (90°C) aids decrosslinking, but increases the rate of depurination and ss-break events that lead to the formation of ss-DNA regions known to favour the misincorporation of nucleotides (A – rule) or generate sequence chimeras. Therefore, milder decrosslinking reactions will reduce the rates of these occurrences. (b) The FFPE process damages DNA bases. The removal of damaged bases by glycosylases improves the quality of readable DNA by removing from the PCR pool damaged template that would otherwise lead to misincorporation of bases leading to SNPs. The product of either glycosylase treatments is AP sites (UDG) or 3′ blocked ends (FPG, Endo VIII) that block polymerase activity. (c) These blocking artefacts are repaired by either an AP endonuclease (AP sites Endo IV), leaving a 3′OH and 5′dRP, or a phosphokinase (3′P & T4 PNK), leaving a 3′OH and a 5′P. Only when ends are repaired (3′OH and 5′P/5′dRP), the DNA repair polymerase (Pol I) able to incorporate nucleotides that are subsequently sealed with a high fidelity DNA ligase (E. coli DNA ligase).
