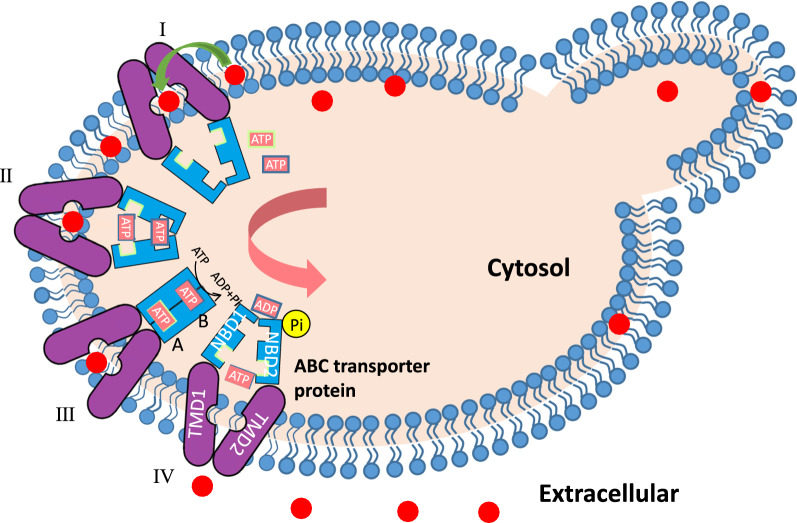
Fig. 1.
The secretion process of β-carotene by ABC transporters in S. cerevisiae. ABC transporters consist of two cytoplasmic nucleotide-binding domains (NBDs, blue) that hydrolyze ATP to drive transport and two transmembrane domains (TMDs, purple) that bind chemical compounds and provide a translocation channel. The specific secretion process of β-carotene is as follows: (I) β-carotene (red circles) is transferred from the cell membrane to the hydrophobic transmembrane domain when the transporter opens inward, (II) then ATP is bound at both sites. (III) ATP binding causes the dimerization of NBDs. The ATP hydrolysis is limited to the canonical one (step III-B), which drives a change from an inward-facing, drug-binding conformation to an outward-facing, drug-releasing conformation. (IV) When β-carotene is secreted out of the cell, the protein was reset in a drug-binding conformation