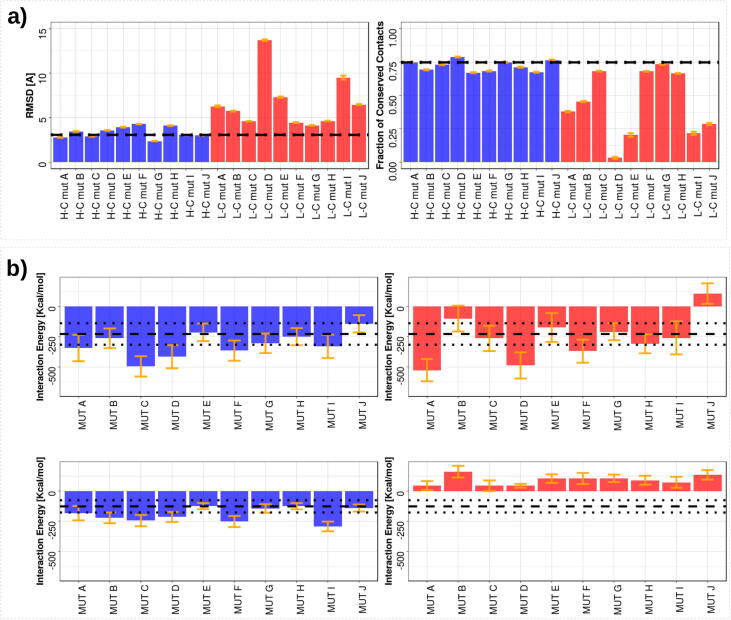
Fig. 5.
Molecular dynamics simulation of the CD71/H-Ft complex. The results in blue and in red are for high and low complementarity mutants, respectively. The mean values presented here are obtained disregarding the initial 25 ns of equilibration. a) The stability analysis of the complexes. In the left panel the mean value of RMSD, with respect to the initial conformation. In the right panel the mean percentage of residue-residue interface contacts conserved with respect to the initial conformation. The black dotted lines represent the mean values observed when the wild type interface is simulated. b) The energetic analysis of the interfaces. The values reported here are averaged on all the MD equilibrium frames. In the top row, we report the mean energy of non-covalent interactions (Coulomb and van der Waals terms) between all the atoms of the CD71 and H-Ft. In the bottom row, we focus on the mean energy exchanged at the interface, i.e., considering only the atoms belonging to residues defined in Fig. 1. The orange line represents one standard deviation interval centered on the mean. The black dotted lines represent the mean and standard deviation of the. wild type case. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)