Abstract
Background
Prematurity contributes greatly to the neonatal mortality burden in sub-Saharan Africa. This study evaluated the proportion of preterm neonatal death, medical conditions at admission, and determinants of mortality of preterm neonates in the neonatal intensive care unit (NICU) of a tertiary hospital in Western Uganda.
Materials and Methods
A prospective cohort study of 351 consecutively enrolled preterm neonates was conducted from March to June 2019. Interviewer-administered questionnaires and physical assessment of neonates were used to obtain socio-demographic and clinical data for mothers and their preterm neonates. Descriptive statistics for participants’ characteristics were generated, while bivariate and multivariate logistic regression models were fitted so as to establish the determinants of mortality outcome. A p-value <0.05 was considered statistically significant.
Results
In-hospital neonatal mortality of 31.6% (95% CI: 26.9–36.7) was noted, with 65.8% of deaths occurring within 72 hours from admission. The most common medical conditions at admission were: hypothermia (67.2%), respiratory distress syndrome (43.0%), small for gestational age (15.7%), and perinatal asphyxia (14.5%). Under multivariate regression modelling, maternal age ≥35 years (AOR: 4.5; 95% CI: 1.35–15.31), no antenatal care (AOR: 4.7; 95% CI: 1.05–21.21), >4 ANC visits (AOR: 5.3; 95% CI: 1.88–15.21), neonatal resuscitation (AOR: 3.4; 95% CI: 1.66–6.82), outborn status (AOR: 2.3; 95% CI: 1.20–4.50), singleton pregnancy (AOR: 3.7; 95% CI: 1.74–7.89), <28 weeks’ gestation (AOR: 12.0; 95% CI: 2.24–64.27), and male sex (AOR: 2.0; 95% CI: 1.04–3.74), respiratory distress syndrome (AOR: 2.6; 95% CI: 1.22–5.70), apnea (AOR: 6.2; 95.5% CI: 1.09–35.38), hypothermia (AOR: 2.3; 95% CI: 1.09–4.92), and small for gestational age (AOR: 4.7; 95% CI: 2.06–10.74) were significantly associated with mortality.
Conclusion and Recommendations
In-hospital mortality of preterm neonates was high. We identified various maternal and neonatal risk factors, indicating a need for stakeholders to enhance efforts towards prevention of preterm-associated complications and optimize facility-based continuum of care.
Keywords: preterm neonate, neonatal intensive care unit, cohort
Background
The World Health Organization (WHO) defines a preterm birth as any birth before 37 completed weeks of gestation.1 Worldwide, an estimated 15 million preterm babies were born in 2010, with over 60% of preterm births occurring in sub-Saharan Africa and South Asia where 9.1 million births are estimated to be preterm annually.2 In 2015, neonatal mortality accounted for 2.7 million (45.1%) of the 5.9 million under-five deaths globally, and complications of prematurity were responsible for approximately one-third of deaths during the first 28 days of life.3 Preterm birth complications are the third leading cause of neonatal mortality in sub-Saharan Africa, after severe neonatal infections and perinatal asphyxia.4 Whereas significant progress has been made to lower under-five mortality, the decline of neonatal mortality has been slow, especially in many low-income countries where only 30% of babies born at 28 to 32 weeks survive and almost all (>90%) those born at <28 weeks die in the first few days of life.3,5,6 Unfortunately, health-care providers and families in many countries still perceive the death of any premature baby as inevitable.6
Preterm babies account for 0.7% of all hospital admissions in Uganda, and yet are responsible for 11.1% of under-five deaths, and 5% of deaths among children of all ages.7 With an estimated neonatal mortality rate of 21.4 [17.2, 26.5] per 1000 live births, stakeholders and health workers need to scale up efforts to address preterm birth and improve the care of preterm babies if Uganda is to achieve the Sustainable Development Goals era target (SDG 3.2) of reducing neonatal mortality rate (NMR) to 12 deaths/1000 live births or less by 2030.8,9
Preterm neonates are physiological immature and have limited compensatory responses to the extra-uterine environment.10 Consequently, they have a higher risk of morbidities such as hypothermia, perinatal asphyxia, respiratory distress syndrome (RDS), apnea, hypoglycemia, jaundice, transient tachypnea, and patent ductus arteriosus, intracranial hemorrhage, necrotizing enterocolitis (NEC), and feeding difficulties.11–14 Preterm neonates are also likely to have a prolonged hospital stay, and their care imposes a high financial burden to families and health facilities.15,16
Several authors have reported an association between maternal age, level of education, occupation, income, place of residence, and antenatal care (ANC), with preterm neonatal mortality.17–20 In other studies, place of delivery, lower gestation age, low birth weight, male sex, low Apgar score at five minutes, prolonged resuscitation, small for gestational age (SGA), and intraventricular hemorrhage, sepsis, NEC, RDS, and hypoglycemia were significantly associated with mortality.14,21–23
Studies regarding preterm newborn mortality in the NICU have been conducted in the Central and Eastern regions of Uganda, but there exists a dearth of information in the Western region. Therefore, the present prospective study was conducted to document the proportion of preterm neonatal death, medical conditions at admission, and determinants of mortality among hospitalized preterm neonates at a tertiary level NICU in Western Uganda.
Materials and Methods
Study Design, Setting, and Population
A hospital-based prospective cohort study involving all inborn and outborn preterm neonates (aged ≤28 days of life) admitted in the NICU of Fort Portal Regional Referral Hospital (FPRRH) was conducted between March 1 and June 30, 2019. The hospital is located in Kabarole district in Western Uganda, approximately 320 kilometers west of Kampala, the capital city of Uganda. It is a public hospital that serves more than eight districts and offers both general and specialized medical services, and is also a training facility for several medical schools within the region. The NICU provides neonatal care services to both inborn and outborn term and preterm neonates, and also admits critically ill preterm neonates who require advanced respiratory and cardiovascular support. The care available for preterm babies includes thermoregulation using six (6) incubators and five (5) radiant warmers, ventilatory support using improvised nasal-bubble continuous positive airway pressure and oxygen therapy using three (3) oxygen concentrators and two (2) cylinders, three (3) phototherapy units for hyperbilirubinemia, intravenous fluid and drug therapy, blood transfusion, and gavage feeding. It also has six (6) cribs, five (5) chairs for Kangaroo mother care (KMC) and two (2) cardiorespiratory monitors. The unit however lacks facilities for mechanical ventilation, exchange blood transfusion, infusion pumps, continuous positive airway pressure (CPAP) machine and parenteral nutrition.
Inclusion and Exclusion Criteria
Preterm neonates admitted to the NICU of FPRRH during the time of data collection were included in the study; while those with no immediate caregiver were excluded.
Sample Size Determination and Sampling Procedure
The sample size of 351 preterm neonates was determined using the modified Daniel’s formula. Based on the assumptions of a proportion of preterm neonatal mortality of 0.354 from a study conducted in Kiwoko hospital, central Uganda;24 95% level of confidence; 5% margin of error; and power of 80%. It was anticipated that no study participant would withdraw from the follow-up. Study participants were consecutively recruited into the study until the required sample size was attained.
Data Collection and Follow-Up
A pre-tested interviewer-administered questionnaire and checklist were used to collect the data. The tool was developed in English, informed by reviewing different published literature, and translated into the local language (Rutooro). The information was collected at the time of admission of the preterm neonates. Mothers of preterm neonates were interviewed by an investigator, who also obtained additional information from the mothers’ clinical records such as antenatal care card, delivery notes, and/or hospital referral form. Using a pre-standardised checklist, the investigator performed a clinical examination of the preterm neonate to establish the medical condition at admission. Random blood glucose was performed for preterm neonates who presented with apnea, lethargy, or convulsions.25 The investigator made a daily follow-up of the neonates in the NICU to identify if they had been discharged; died; referred; or lost to follow-up. Follow-up was continued until discharge or death of the neonate, up to a maximum of 28 days of life, whichever came first.
Study Variables
The mortality of preterm neonates was the dependent variable. Regarding the independent variables for this study, data were collected on maternal socio-demographic status, maternal economic status, obstetric, and medical characteristics, and medical diagnosis at admission of preterm neonates. These variables included maternal age, district of residence, marital status, religion, level of education, occupation, monthly income, and parity, history of abortion and/or preterm birth, ANC visits, human immunodeficiency virus (HIV) status, pregnancy, and labor complications. In addition, information on place of delivery, mode of delivery, gestation type, type of birth attendant, and antenatal corticosteroid use, neonatal postnatal age at admission, gender, gestational age, birth weight, 5-minute APGAR score, and resuscitation history were collected.
Data Quality Control
Pre-testing of the questionnaires was done using ten (10) caretakers of preterm neonates in an NICU of similar status to the study participants and enabled clarifications of questions prior to approval of the final version of the questionnaire that was finally implemented. This information was not included in the data analysis. Only a well-trained clinician assessed the patients’ presenting problem(s), perinatal events including gestational age using the mother’s last normal menstrual period (LNMP) or the new Ballard score (for neonates aged 3 days or less) for mothers who do not know the date of the first day of their LNMP, Apgar score, birth weight, and physical examination. The digital weighing scale was calibrated and validated daily using the same object of known weight, and was placed on a firm flat surface before each weight measurement. Standard treatment protocols were used by all doctors and nurses to ensure uniformity in patient care. Data were checked for completeness, accuracy, clarity, and consistency before the interview was terminated. Proper coding and categorization of data were maintained for the quality of the data to be analyzed, and double data entry was used to ensure reliability of data entry and compared with the original data.
Statistical Analysis
Data were entered in a Microsoft Excel 2016 database and all statistical analysis was performed using Stata® software (v.13, College Station, Texas, USA). Maternal and preterm neonatal characteristics were described using means for continuous variables and proportions for categorical variables. The primary outcome of the study was in-hospital mortality of preterm neonates within the first 28 days of hospital admission. The proportion of preterm neonates who died during hospitalization was calculated at 3 time points: less than 24 hours, 24 hours and less than 72 hours, and 72 hours or more since admission. The survival time was calculated in hours between the date of admission and the date of death. The proportions of preterm neonates admitted in the NICU that had each type of medical condition was calculated. Using Chi-square test, the frequencies of each medical condition as diagnosed at admission were compared across gestational age categories. A significance level of 5% was used. In bivariate analysis, Chi-square test and logistic regression were used to compare maternal or preterm neonatal factors with death of preterm neonates. Crude odds ratios with their corresponding 95% CI were obtained. A variable was considered significant in this analysis if it had a p<0.05. All factors with p-value <0.2 and those which are biologically plausible with in-hospital death of preterm neonates were considered in the multivariate analysis which was performed to control confounding. Assumptions for use of multiple logistic regression, for example, the absence of multicollinearity among the independent variables, were explored. A manual back-ward stepwise selection method was used in establishing the final multivariate analysis model with factors that bear an independent significant association with death of preterm neonates. The factors in the final multivariate model were then reported together with their adjusted odds ratios and 95% confidence intervals. A variable was considered significant in this analysis if it had a p<0.05.
Ethical Considerations
We obtained ethical approval from the Research Ethics Review Committee of Kampala International University (reference number UG-REC-023/201,907). Permission to execute the study was granted by the Hospital Director of FPRRH. Consent for participants aged less than 18 years was approved by the ethics committee to provide written informed consent on their own behalf, and the study was conducted in accordance with the ethical principles and regulations set in the Declaration of Helsinki, regarding biomedical research involving human subjects.
Operational Definitions
In this study, gestational age was calculated from the LNMP to the date of delivery. If the mother did not remember her LNMP, gestational age was estimated using the new Ballard score, for preterm neonates who were less than 72 hours of age.26 The preterm neonates’ medical conditions at admission were defined as follows: temperature instability as an axillary temperature of less than 36.0°C (hypothermia) or higher than 37.5°C (fever); hypoglycemia as glucose below 40 mg/dL (<2.2 mmol/l) in a capillary or venous sample, using a glucometer, and hyperbilirubinemia as yellowing of the eyes and/or body requiring phototherapy based on the WHO criteria.25 Apnea was defined as a cessation of respiration for more than 20 seconds or any duration if accompanied by cyanosis, bradycardia (<100 beats per minute) or pallor;27 while perinatal asphyxia was diagnosed when a neonate had an Apgar score of 6 or less at five minutes.26,28 For the diagnosis of RDS, being preterm with any of the following signs and symptoms; fast breathing, grunting, subcostal and intercostal recession, cyanosis and reduced air entry in bilateral lung fields starting in the first four hours of life.29 A clinical diagnosis of sepsis was made according to the WHO’s Integrated Management of Childhood Illness (IMCI) guidelines, defined as the presence of the following: history of difficulty feeding, history of convulsions, movement only when stimulated, respiratory rate of ≥60 breaths per minute, severe chest retractions, or a temperature of ≥37.5°C or ≤35.5°C.25 Anthropometric measurements were interpreted using the Lubchenco curve.30
Results
Out of 352 eligible preterm neonates admitted in the NICU during the study recruitment period, 351 were included in the study, hence a response rate of 99.7%. One neonate died before consent could be obtained and none was lost to follow-up.
Socio-Demographic and Economic Characteristics of Mothers
The mean maternal age was 25.6 ± 5.9 years. The majority (86.3%) of the mothers were aged between 18 and 34 years, 91.4% were married, and 58.4% had attained primary level education. Other characteristics are shown in Table 1.
Table 1.
Baseline Socio-Economic and Demographic Characteristics of the Mothers of Preterm Neonates
| Variables | Frequency (N=351) | Percentage |
|---|---|---|
| Maternal age (years) | ||
| <18 | 20 | 5.7 |
| 18–24 | 150 | 42.7 |
| 25–34 | 153 | 43.6 |
| ≥35 | 28 | 8.0 |
| District of residence | ||
| Kabarole | 226 | 64.4 |
| Ntoroko | 11 | 3.1 |
| Bundibugyo | 25 | 7.1 |
| Kyenjojo | 32 | 9.1 |
| Kasese | 10 | 2.9 |
| Kamwenge | 7 | 2.0 |
| Kyegegwa | 21 | 6.0 |
| Bunyangabu | 17 | 4.8 |
| Other | 2 | 0.6 |
| Marital status | ||
| Single | 30 | 8.6 |
| Married | 321 | 91.4 |
| Religion | ||
| Catholic | 218 | 62.1 |
| Anglican | 72 | 20.5 |
| Muslim | 18 | 5.1 |
| Pentecostal | 22 | 6.3 |
| Seventh Day Adventist | 21 | 6.0 |
| Education level | ||
| No formal education | 28 | 8.0 |
| Primary | 205 | 58.4 |
| Secondary | 109 | 31.0 |
| Tertiary | 9 | 2.6 |
| Occupation (n=339) | ||
| Peasant | 209 | 61.7 |
| Business | 111 | 32.7 |
| Formal employment | 19 | 5.6 |
| Monthly income | ||
| None | 49 | 14.0 |
| <100,000 | 235 | 67.0 |
| ≥100,000 | 67 | 19.0 |
Obstetric Characteristics and Medical Conditions Among Mothers
As shown in Table 2, primiparous mothers were 68 (19.4%), multiparas were 200 (57.0%), and grand multiparas were 83 (23.6%). Majority (90.3%) of mothers had no history of abortion or previous preterm birth (92.6%). Twenty-one (6.0%) did not attend ANC. Among those who attended ANC, 53.2% had their first visit at ≥4 months gestation. Two hundred preterm neonates (57.0%) were inborn, 229 (65.2%) were singleton pregnancies and the majority (90.9%) were delivered by skilled health workers who included doctors, midwives and nurses. Two hundred and fifty-three deliveries (72.1%) were vaginal, most mothers, 319 (90.9%) did not receive prenatal corticosteroids, and 46 (13.1%) were positive for HIV. Pregnancy and labor complications were reported in 134 (38.2%), and 66 (18.8%) of the mothers, respectively. Complications included PROM, urinary tract infections, pre-eclampsia, eclampsia, malaria, and antepartum hemorrhage.
Table 2.
Baseline Obstetric Characteristics and Medical Conditions Among Mothers of Preterm Neonates
| Variables | Frequency (N=351) | Percentage |
|---|---|---|
| Parity | ||
| 1 | 68 | 19.4 |
| 2–4 | 200 | 57.0 |
| ≥5 | 83 | 23.6 |
| History of abortion | ||
| Yes | 34 | 9.7 |
| No | 317 | 90.3 |
| Previous preterm birth | ||
| Yes | 26 | 7.4 |
| No | 325 | 92.6 |
| Number of ANC visits | ||
| None | 21 | 6.0 |
| 1–2 | 124 | 35.3 |
| 3–4 | 173 | 49.3 |
| >4 | 33 | 9.4 |
| First ANC visit | ||
| <4 months | 160 | 46.8 |
| ≥4 months | 182 | 53.2 |
| HIV status | ||
| Positive | 46 | 13.1 |
| Negative | 305 | 86.9 |
| Pregnancy complications | ||
| Yes | 134 | 38.2 |
| No | 217 | 61.8 |
| Labor complications | ||
| Yes | 66 | 18.8 |
| No | 285 | 81.2 |
| Place of delivery | ||
| Inborn | 200 | 57.0 |
| Outborn | 151 | 43.0 |
| Mode of delivery | ||
| Vaginal | 253 | 72.1 |
| Cesarean section | 98 | 27.9 |
| Type of gestation | ||
| Singleton | 229 | 65.2 |
| Multiple | 122 | 34.8 |
| Type of birth attendant | ||
| Skilled | 319 | 90.9 |
| Unskilled | 32 | 9.1 |
| Antenatal corticosteroids | ||
| Yes | 32 | 9.1 |
| No | 319 | 90.9 |
Characteristics of Preterm Neonates
Baseline characteristics of preterm neonates are presented in Table 3. The mean postnatal age of preterm neonates at admission to the NICU was 12.6 ± 26.4 hours. The majority (85.2%) had a postnatal age of less than 24 hours at admission. Females comprised 51.0% (179/351) of admissions. Basing on gestational age, 35 (10.0%) were extremely preterm (<28 weeks of gestation), 102 (29.0%) very preterm (28–31 weeks), 82 (23.4%) moderate preterm (32–33 weeks), and 132 (37.6%) were late preterm (34–36 weeks). Most neonates (68.1%) had a birth weight of 1.5–2.4 kg, with a mean weight of 1.72 ± 0.49 kg. Among the 314 preterm neonates with a documented 5-minute Apgar score, 85.0% had a 5-minute Apgar score of ≥7. Resuscitation was performed for 26.5% of preterm neonates studied.
Table 3.
Baseline Characteristics of Preterm Neonates
| Variables | Frequency (N=351) | Percentage |
|---|---|---|
| Postnatal age at admission (days) | ||
| <1 | 299 | 85.2 |
| ≥1 | 52 | 14.8 |
| Gender | ||
| Females | 179 | 51.0 |
| Males | 172 | 49.0 |
| Gestational age (weeks) | ||
| <28 | 35 | 10.0 |
| 28–31 | 102 | 29.0 |
| 32–33 | 82 | 23.4 |
| 34–36 | 132 | 37.6 |
| Birth weight (kg) | ||
| <1 | 29 | 8.3 |
| 1–1.4 | 74 | 21.1 |
| 1.5–2.4 | 239 | 68.1 |
| ≥2.5 | 9 | 2.5 |
| APGAR score at 5 minutes* | ||
| <7 | 47 | 15.0 |
| ≥7 | 267 | 85.0 |
| Resuscitation | ||
| Yes | 93 | 26.5 |
| No | 258 | 73.5 |
Note: *Apgar score at 5 minutes was documented for 314 preterm neonates.
Medical Conditions Among Preterm Neonates at Admission
Overall, the leading medical conditions among preterm neonates at admission were hypothermia (67.2%), RDS (43.0%), SGA (15.7%) and perinatal asphyxia (14.5%). Congenital anomalies included gastroschisis (n=2), omphalocele (n=2), cleft lip and palate (n=1), hypospadias (n=1), spina bifida (n=1), polydactyly (n=7) and microcephaly (n=1). Table 4 shows the stratification of medical conditions at admission by gestational age of preterm neonates. The prevalence of RDS, apnea, and hypothermia, significantly decreased with increasing gestational age, p<0.001, while the prevalence of sepsis was higher among neonates with higher gestational age, p<0.039.
Table 4.
Distribution of Medical Conditions at Admission by Gestational Age of Preterm Neonates
| Medical Condition | All Neonates (N=351) | Gestation Age (Weeks) | P-value | |||
|---|---|---|---|---|---|---|
| <28 (n=35) |
28–31 (n=102) |
32–33 (n=82) |
34–36 (n=132) |
|||
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| NEC | 2 (0.57) | 1 (2.9) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0.190 |
| RDS | 151 (43.0) | 26 (74.3) | 73 (71.6) | 36 (43.9) | 16 (12.1) | <0.001 |
| Jaundice | 8 (2.3) | 1 (2.9) | 1 (1.0) | 1 (1.2) | 5 (3.8) | 0.460 |
| Perinatal asphyxia | 51 (14.5) | 8 (22.9) | 13 (12.8) | 11 (13.4) | 19 (14.4) | 0.513 |
| HDNB | 6 (1.7) | 0 (0.0) | 3 (2.9) | 0 (0.0) | 3 (2.3) | 0.361 |
| SGA | 55 (15.7) | 6 (17.1) | 21 (20.6) | 6 (7.3) | 22 (16.7) | 0.096 |
| Apnea | 32 (9.1) | 23 (65.7) | 9 (8.8) | 0 (0.0) | 0 (0.0) | <0.001 |
| Hypothermia | 236 (67.2) | 33 (94.3) | 80 (78.4) | 51 (62.2) | 72 (54.6) | <0.001 |
| Sepsis | 29 (8.3) | 1 (2.9) | 6 (5.9) | 4 (4.9) | 18 (13.6) | 0.039 |
| Hypoglycemia | 5 (1.4) | 0 (0.0) | 1 (1.0) | 1 (1.2) | 3 (2.3) | 0.717 |
| Congenital anomalies | 15 (4.3) | 2 (5.7) | 2 (2.0) | 3 (3.7) | 8 (6.1) | 0.454 |
Notes: Frequencies and percentages do not add-up to 100% because some preterm neonates had more than one medical condition at admission. Percentages reflect the number of neonates with a particular medical condition who were recorded and analyzed as a proportion of the total number of neonates in a particular category.
Abbreviations: NEC, necrotizing enterocolitis; RDS, respiratory distress syndrome; HDNB, hemorrhagic disease of the newborn; SGA, small for gestational age.
Outcomes of Preterm Neonates
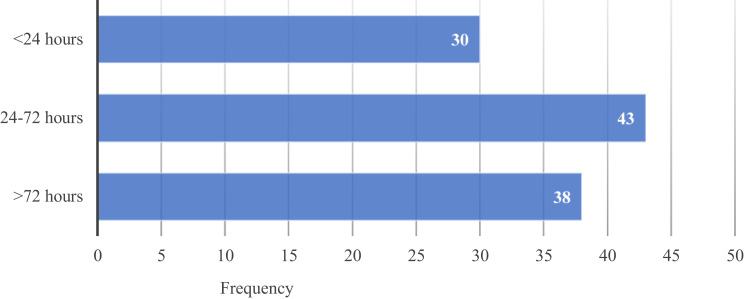
In this study, 31.6% (95% CI: 26.9–36.7) of admitted preterm neonates died, and 68.4% (95% CI: 63.3–73.1) survived. Gestational age was inversely related to mortality (p<0.004). Preterm neonates who survived included those who recovered and were discharged (97.1%); left against medical advice (0.4%) or were referred to a national referral hospital (2.5%). Two-thirds (73/111) of preterm neonatal deaths occurred less than 72 hours from admission (Figure 1).
Figure 1.
Distribution of preterm neonatal deaths based on duration of hospital stay.
Risk Factors for Mortality of Preterm Neonates
Preterm neonates whose mothers were aged 35 years and above were 4.5 times more likely to die, when compared to those whose mothers were aged 25–34 years (AOR: 4.5; 95% CI: 1.35–15.31). Preterm neonates whose mothers did not enroll for ANC had 4.7 times more likelihood of death (AOR: 4.7; 95% CI: 1.05–21.21), while those whose mothers attended more than four ANC visits were 5.3 times more likely to die than those whose mothers attended 3–4 visits (AOR: 5.3; 95% CI: 1.88–15.21). Compared to inborn preterm neonates, outborns had a 2.3 times more likelihood of death (AOR: 2.3; 95% CI: 1.20–4.50). In addition, singleton deliveries had a 3.7 times higher odds of mortality compared to multiple gestations (AOR: 3.7; 95% CI: 1.74–7.89); whereas extremely preterm neonates had a 12 times higher likelihood of death compared to late preterm neonates (AOR: 12.0; 95% CI: 2.24–64.27). In this study, male neonates were 2 times more likely to die compared to females (AOR: 2.0; 95% CI: 1.04–3.74). The odds of mortality were 3.4 times higher among preterm neonates who were resuscitated at birth compared to those who were not resuscitated (AOR: 3.4; 95% CI: 1.66–6.82). Similarly, preterm neonates who were diagnosed with RDS (AOR: 2.6; 95% CI: 1.22–5.70), SGA (AOR: 4.7; 95% CI: 2.06–10.74), apnea (AOR: 6.2; 95% CI: 1.09–35.38), or hypothermia (AOR: 2.3; 95% CI: 1.09–4.92) at admission were likely to die. Details are shown in Table 5.
Table 5.
Results of Bivariate and Multivariate Logistic Regression Analyses of Factors Independently Associated with Mortality of Preterm Neonates
| Variables | Survived (n=240) | Died (n-111) | COR (95% CI) | AOR (95% CI) | P-value |
|---|---|---|---|---|---|
| Frequency (%) | Frequency (%) | ||||
| Maternal age (years) | |||||
| <18 | 7 (35.0) | 13 (65.0) | 5.1 (1.89–13.59) | 3.2 (0.87–11.96) | 0.079 |
| 18–24 | 106 (70.7) | 44 (29.3) | 1.1 (0.69–1.87) | 1.3 (0.63–2.70) | 0.467 |
| 25–34 | 112 (73.2) | 41 (26.8) | 1 | 1 | - |
| ≥35 | 15 (53.6) | 13 (46.4) | 2.4 (1.04–5.39) | 4.5 (1.35–15.31) | 0.014 |
| Number of ANC visits | |||||
| None | 4 (19.0) | 17 (81.0) | 22.9 (7.18–73.60) | 4.7 (1.05–21.21) | 0.043 |
| 1–2 | 69 (55.7) | 55 (44.3) | 4.3 (2.51–7.41) | 1.9 (0.90–3.86) | 0.094 |
| 3–4 | 146 (84.4) | 27 (15.6) | 1 | 1 | - |
| >4 | 21 (63.6) | 12 (36.4) | 3.1 (1.36–7.01) | 5.3 (1.88–15.21) | 0.002 |
| Place of delivery | |||||
| Out-born | 85 (56.3) | 66 (43.7) | 2.7 (1.68–4.25) | 2.3 (1.20–4.50) | 0.013 |
| In-born | 155 (77.5) | 45 (22.5) | 1 | 1 | - |
| Type of gestation | |||||
| Singleton | 147 (64.2) | 82 (35.8) | 1.8 (1.09–2.94) | 3.7 (1.74–7.89) | 0.001 |
| Multiple | 93 (76.2) | 29 (23.8) | 1 | 1 | - |
| Gestational age (weeks) | |||||
| <28 | 3 (8.6) | 32 (91.4) | 53.3 (14.99–189.70) | 12.0 (2.24–64.27) | 0.004 |
| 28–31 | 63 (61.8) | 39 (38.2) | 3.1 (1.69–5.68) | 1.44 (0.58–3.59) | 0.433 |
| 32–33 | 64 (78.0) | 18 (22.0) | 1.4 (0.70–2.82) | 1.4 (0.57–3.36) | 0.469 |
| 34–36 | 110 (83.3) | 22 (16.7) | 1 | 1 | - |
| Gender | |||||
| Male | 110 (64.0) | 62 (36.0) | 1.5 (0.95–2.35) | 2.0 (1.04–3.74) | 0.038 |
| Female | 130 (72.6) | 49 (27.4) | 1 | 1 | |
| Resuscitation | |||||
| Yes | 45 (48.4) | 48 (51.6) | 3.3 (2.01–5.42) | 3.4 (1.66–6.82) | 0.001 |
| No | 195 (75.6) | 63 (24.4) | 1 | 1 | - |
| RDS | |||||
| Yes | 84 (55.6) | 67 (44.4) | 2.8 (1.78–4.49) | 2.6 (1.22–5.70) | 0.014 |
| No | 156 (78.0) | 44 (22.0) | 1 | 1 | - |
| SGA | |||||
| Yes | 25 (45.5) | 30 (54.5) | 3.2 (1.77–5.74) | 4.7 (2.06–10.74) | <0.001 |
| No | 215 (72.6) | 81 (27.4) | 1 | 1 | - |
| Apnea | |||||
| Yes | 2 (6.3) | 30 (93.7) | 44.1 (10.30–188.53) | 6.2 (1.09–35.38) | 0.039 |
| No | 238 (74.6) | 81 (25.4) | 1 | 1 | - |
| Hypothermia | |||||
| Yes | 142 (60.2) | 94 (39.8) | 3.8 (2.14–6.80) | 2.3 (1.09–4.92) | 0.029 |
| No | 98 (85.2) | 17 (14.8) | 1 | 1 | - |
Abbreviations: AOR, adjusted odds ratio; COR, crude odds ratio; CI, confidence interval; RDS, respiratory distress syndrome; SGA, small for gestational age.
Discussion
This study evaluated the proportion of neonatal death, medical conditions at admission, and determinants of mortality among preterm neonates in a tertiary level NICU in Western Uganda. Overall, nearly one-third (31.6%) of the admitted preterm neonates died. This is consistent with the findings in the University of Gondar Comprehensive Specialized Hospital in Ethiopia (28.8%),14 Alex Ekwueme Federal University Teaching Hospital, Nigeria (31.5%),31 and Jimma University Specialized Hospital (34.9%) in Ethiopia.32 This is higher than 27.4%, 24.0%, 22.1%, and 11.4% that were reported in Fatemieh hospital, Iran,33 University of Nigeria Teaching Hospital in Nigeria,34 Mulago National Referral Hospital in central Uganda,35 and Nairobi hospital in Kenya36 respectively. In contrast, the mortality was lower than 37.9% in Dessie Referral Hospital in Ethiopia,21 and 35.2% in Kiwoko hospital in central Uganda.24 This marked disparity in mortality could be due to inequalities in neonatal care. Some preterm neonates could have been treated in more specialized and equipped facilities compared to the suboptimal care provided in inadequately equipped NICUs.
With respect to the timing of mortality, the results indicated that 27%, and approximately two-thirds (65.8%) of preterm neonates die within the first 24 hours and 72 hours of admission, respectively. These findings are similar to those by other studies.37,38 The first 7 days are the most critical period of a neonate’s life,26 which warrants close observation.
With decreasing frequency, medical conditions at admission included hypothermia (67.2%), RDS (43.0%), SGA (15.7%), perinatal asphyxia (14.5%), apnea (9.1%), sepsis (8.3%), congenital anomalies (4.3%), jaundice (2.3%), and hemorrhagic disease of the newborn (1.7%), hypoglycemia (1.4%), and necrotizing enterocolitis (0.57%). A study in Ethiopia also found that hypothermia, hypoglycemia, jaundice, perinatal asphyxia, respiratory distress, and sepsis were common among preterm neonates at admission.14 In Bangladesh, a retrospective study reported jaundice (14.4%), sepsis (6.2%), transient tachypnea (2.3%), RDS (2.9%) and perinatal asphyxia (1.2%) as common complications,12 while in Tanzania, a prospective cohort study reported hypothermia (37.4%), followed by RDS (32.3%), infection (9.1%), perinatal asphyxia (7.1%), and necrotizing enterocolitis (2.0%).39 These wide variations in proportions may be due to differences in diagnostic criteria, and inclusion of varying gestational age subcategories of preterm neonates.
The factors associated with mortality of preterm neonates are multifactorial and diverse. In the current study, maternal age ≥35 years was associated with mortality of hospitalized preterm neonates, which corresponds to the findings of several studies.17,18 Furthermore, lack of ANC, and attending more than four ANC visits had a significant association with mortality. Generally, studies have shown a protective effect of ANC attendance against neonatal mortality, although regional differences have been reported.40,41 Lack of ANC or less than eight ANC visits is associated with increased likelihood of preterm birth, and its associated complications.42 It is noteworthy, however, that there were few mothers who did not attend ANC, and those who attended more than four ANC visits. Therefore, the study may lack statistical power to identify an effect, also reflected in the wide confidence interval of this outcome at multivariate analysis. It is well recognized that maternal health during pregnancy significantly impacts on neonatal survival. Moreover, it is also clear that unskilled ANC provision and not receiving ANC during the first twelve weeks of gestation may result in failure to detect early pregnancy complications, and poor neonatal outcomes.40,43
This study revealed that babies from singleton gestations were more likely to die than those from multiple gestations. In contrast to this finding, several authors have reported a significant association of multiple gestations with preterm mortality.44,45 A retrospective study of extreme preterm neonates admitted to NICUs in the Australian and New Zealand Neonatal Network reported an insignificant difference in the odds of poor outcomes in multiple and singleton deliveries in more recent years,46 while a prospective study in Tanzania found that twin preterm neonates were less likely to die in the NICU.39 However, this was an in-hospital study that was conducted over a short follow-up period. Another possible explanation for this difference could be because neonates of multiple gestations are more likely to have a lower birth weight, and thus require hospitalization.47
Similar to other studies, mortality of preterm neonates was inversely proportional to gestational age.13,22,48,49 Lower gestational age was significantly associated with increased likelihood of having complications such as RDS, apnea, and hypothermia. This suggests that prevention of these complications may result in increased survival of babies born too soon.
Neonates who were resuscitated at birth were more likely to die. In the current study, preterm neonates who were resuscitated were also most likely to have had a low 5-minute Apgar score, which is a sensitive indicator for the quality of resuscitation provided. Moreover, many health facilities in developing countries are inadequately prepared, as demonstrated by the lack of essential equipment for neonatal resuscitation.50 Our findings are congruent with studies in Ethiopia,32 Iran,33 and Nigeria.51
We found that outborn babies were 2.3 times more likely to die compared to their inborn counterparts. In keeping with these findings, studies in Uganda,24 Nigeria,51 and South Africa52 demonstrated that outborn preterm neonates were more likely to die compared to inborns. This disproportionate mortality is most likely attributable to the fact that outborn preterm neonates are more likely to be exposed to certain risk factors for mortality such as hypothermia during transportation, delays in accessing appropriate healthcare, and to be delivered by non-skilled birth attendants. This finding may suggest that mothers in preterm labor are more likely to have their babies survive when referred to a tertiary health facility for delivery.
With respect to gender, this study revealed that male sex is a predictor of mortality, with males being 2 times more likely to die compared to females. Indeed, several multicentric studies have consistently reported a significant survival advantage of female over male neonates.44,53 This was also demonstrated in a study in Brazil, where male preterm neonates had a 2.99 times higher likelihood of mortality compared to females.37 In New South Wales and Australia, male preterm neonates had 1.29 times higher risk of death before discharge as compared to females.54 This vulnerability may be influenced by the innate sex-specific differences in genetic, hormonal, and immunological makeup.55
This study reiterates the contribution of apnea, RDS, hypothermia, and SGA to preterm neonatal mortality. This is similar to studies in Nepal,11 New South Wales and Australian Capital Territory,23 Iran,33 Tanzania,39 and Ethiopia.48 The preponderance of RDS and hypothermia in this cohort may be explained by the suboptimal use of prenatal corticosteroids, lack of delivery room continuous positive airway pressure, exogeneous surfactant, and under-utilization of mechanisms to keep preterm neonates warm during transportation to the NICU.
Strengths and Limitations
This study was conducted prospectively and included preterm neonates of all gestation age subcategories. However, the findings of this study should be interpreted with consideration of its limitations. This was a single centre study, and diagnosis of some medical conditions at admission was solely based on the clinical symptoms and signs, given the non-availability of investigations such as routine cranial ultrasound scan, echocardiography, blood culture, arterial blood gases, and blood pH during the data collection period. Besides, some preterm neonates developed other complications during the course of hospitalization, which could have significantly contributed to their demise. Preterm neonates were also not followed up after discharge from the NICU.
Conclusions and Recommendations
This study found a high proportion of preterm neonatal mortality in the NICU. Nearly two-thirds of preterm neonates died within the first 72 hours of admission, and the most common medical conditions at admission were hypothermia, RDS, SGA, and perinatal asphyxia. Neonatal mortality was significantly influenced by maternal and neonatal factors such as maternal age ≥35 years, lack of ANC attendance, >4 ANC visits, and singleton delivery. Other contributory factors included resuscitation at birth, outborn status, low gestational age, and male gender. The main causes of mortality were apnea, hypothermia, RDS, and SGA.
Based on these findings, special attention should be directed towards preterm-associated complications, many of which are preventable. Our study also points to the need for intensive monitoring of neonates during the first 72 hours of admission to the NICU. In order to improve preterm newborn survival, stakeholders should reinforce specific strategies that target improvements in facility-based continuum of care such as quality ANC, neonatal resuscitation, respiratory support, and thermal care. Hospitals need to liaise with catchment institutions and communities to promote early recognition and referral of high-risk preterm neonates.
Acknowledgments
The authors acknowledge Dr Khauka Musa and all the staff of the NICU of Fort Portal Regional Referral Hospital for the invaluable support provided during data collection.
Funding Statement
There was no special funding for the study.
Abbreviations
ANC, antenatal care; AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; HDNB, hemorrhagic disease of the newborn; HIV, human immunodeficiency virus; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; OR, odds ratio; RDS, respiratory distress syndrome; SGA, small for gestational age; WHO, World Health Organisation.
Data Sharing Statement
The analysed dataset will be shared by the corresponding author upon request.
Author Contributions
All authors made substantial contributions to the conception and design of the study, acquisition of data, or data analysis and interpretation, took part in drafting the article or revising it critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests for this work.
References
- 1.WHO, March of Dimes, PMNCH, Save the Children. Born Too Soon: The Global Action Report on Preterm Birth. In: Howson CP, Kinney MV, Lawn JE, Eds. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Blencowe H, Cousens S. Review: addressing the challenge of neonatal mortality. Trop Med Int Health. 2013;18(3):303–312. doi: 10.1111/tmi.12048 [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed I, Ali SM, Amenga-Etego S; The Alliance for Maternal and Newborn Health Improvement (AMANHI) mortality study group. Population-based rates, timing, and causes of maternal deaths, stillbirths, and neonatal deaths in south Asia and sub-Saharan Africa: a multi-country prospective cohort study. Lancet Glob Health. 2018;6:1297–1308. doi: 10.1016/S2214-109X(18)30385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arunda MO, Agardh A, Asamoah BO. Survival of low birthweight neonates in Uganda: analysis of progress between 1995 and 2011. BMC Pregnancy Childbirth. 2018;18:189. doi: 10.1186/s12884-018-1831-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn JE, Davidge R, Paul VK, et al. Born too soon: care for the preterm baby. Reprod Health. 2013;10:S5. doi: 10.1186/1742-4755-10-S1-S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ministry of Health. The Republic of Uganda: Ministry of Health; Annual Health Sector Performance Report. Financial Year 2017/18. 2018. [Google Scholar]
- 8.United Nations Children’s Fund. Every child alive, the urgent need to end newborn deaths. Switzerland; 2018Available from: https://data.unicef.org/resources//every_child_alive_the_urgent_need_to_end_newborn_deaths. Accessed June2, 2020. [Google Scholar]
- 9.World Health Organization. World health statistics 2018: monitoring health for the SDGs, sustainable development goals. World Health Organization; 2018. Available from: http://www.who.int/iris/handle/10665/272596. [Google Scholar]
- 10.Engle WA, Tomashek KM, Wallman C. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120(6):1390–1401. doi: 10.1542/peds.2007-2952 [DOI] [PubMed] [Google Scholar]
- 11.Ashish K, Wrammert J, Nelin V, Ewald U, Clark R, Målqvist M. Level of mortality risk for babies born preterm or with a small weight for gestation in a tertiary hospital of Nepal. BMC Public Health. 2015;15:877. doi: 10.1186/s12889-015-2232-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begum LN, Ahmed F, Haq K, Mallick LL. Clinical outcome of the late preterm infants. Bangabandhu Sheikh Mujib Medi Univ J. 2017;10:132–134. doi: 10.3329/bsmmuj.v10i3.32922 [DOI] [Google Scholar]
- 13.Fajolu I, Akintan PE, Ezenwa B, Ezeaka VC. Survival of extremely preterm neonates in a resource-limited setting. Iran J Neonatol. 2019;10(3):32–37. doi: 10.22038/ijn.2019.38772.1611 [DOI] [Google Scholar]
- 14.Yismaw AE, Tarekegn AA. Proportion and factors of death among preterm neonates admitted in University of Gondar comprehensive specialized hospital neonatal intensive care unit, Northwest Ethiopia. BMC Res Notes. 2018;11:867. doi: 10.1186/s13104-018-3970-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunle-olowu OE, Peterside O, Adeyemi OO. Prevalence and outcome of preterm admissions at the neonatal unit of a tertiary health centre in Southern Nigeria. Open J Pediatr. 2014;4:67–75. doi: 10.4236/ojped.2014.41009 [DOI] [Google Scholar]
- 16.Tongo OO, Orimadegun AE, Ajayi SO, Akinyinka OO. The economic burden of preterm/very low birth weight care in Nigeria. J Trop Pediatr. 2008;55(4):262–264. doi: 10.1093/tropej/fmn107 [DOI] [PubMed] [Google Scholar]
- 17.Kozuki N, Lee ACC, Silveira MF, et al. The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health. 2013;13:S2. doi: 10.1186/1471-2458-13-S3-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mashako R, Ngbonda D, Alworong’a O, et al. Predictive factors of neonatal mortality in intensive neonatal care unit at Goma Eastern Democratic Republic of Congo. J Pediatr Neonatal Care. 2019;9(2):58–61. doi: 10.15406/jpnc.2019.09.00376 [DOI] [Google Scholar]
- 19.Mekonnen Y, Tensou B, Telake DS, Degefie T, Bekele A. Neonatal mortality in Ethiopia: trends and determinants. BMC Public Health. 2013;13:483. doi: 10.1186/1471-2458-13-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Dai Q, Xu Y, et al. Time trends and risk factor associated with premature birth and infants deaths due to prematurity in Hubei Province, China from 2001 to 2012. BMC Pregnancy Childbirth. 2015;15:329. doi: 10.1186/s12884-015-0767-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayele MW, Yitayih G, Emshaw S, et al. Treatment outcomes and associated factors of preterm birth of neonates admitted in intensive care unit of Dessie Referral Hospital, North Central Ethiopia. J Nurs Care. 2019;8(2):484. [Google Scholar]
- 22.Kong X, Xu F, Wu R, et al. Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: a multicenter survey in China from 2013 to 2014. BMC Pediatr. 2016;16:174. doi: 10.1186/s12887-016-0716-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindler T, Koller-smith L, Lui K, et al. Causes of death in very preterm infants cared for in neonatal intensive care units: a population-based retrospective cohort study. BMC Pediatr. 2017;17:59. doi: 10.1186/s12887-017-0810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedstrom A, Ryman T, Otai C, et al. Demographics, clinical characteristics and neonatal outcomes in a rural Ugandan NICU. BMC Pregnancy Childbirth. 2014;14:327. doi: 10.1186/1471-2393-14-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organisation. Pocket book of hospital care for children: guidelines for the management of childhood illness (2nd ed.); 2013. Geneva, Switzerland: Available from: https://www.ncbi.nlm.nih.gov/books/NBK154447/pdf/Bookshelf_NBK154447.pdf. [PubMed] [Google Scholar]
- 26.Hadgu FB, Gebretsadik LG, Mihretu HG, Berhe AH. Prevalence and factors associated with neonatal mortality at Ayder Comprehensive Specialized Hospital, Northern Ethiopia. A cross-sectional study. Pediatr Health Med Therap. 2020;11:37. doi: 10.2147/PHMT.S235591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eichenwald EC. Committee on fetus and newborn. Apnea of prematurity. Pediatrics. 2016;137(1):e20153757. doi: 10.1542/peds.2015-3757 [DOI] [PubMed] [Google Scholar]
- 28.WHO. Guidelines Approved by the Guidelines Review Committee. Guidelines on Basic Newborn Resuscitation. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 29.Tochie JN, Choukem S-P, Langmia RN, Barla E, Koki-Ndombo P. Neonatal respiratory distress in a reference neonatal unit in Cameroon: an analysis of prevalence, predictors, etiologies and outcomes. Pan Afr Med J. 2016;24:152. doi: 10.11604/pamj.2016.24.152.7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lubchenco L, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from live born birth weight data at 24 to 42 weeks of gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- 31.Onyema MC, Obi VO, Nwafor JI, Oliobi CW, Uche-nwidagu BN, Nweke AN. Profiling the Socio-demographic characteristics and outcome of preterm delivery in Alex Ekwueme Federal University Teaching Hospital Abakaliki. Open J Obstet Gynecol. 2019;9:1168–1177. doi: 10.4236/ojog.2019.98113 [DOI] [Google Scholar]
- 32.Wesenu M, Kulkarni S, Tilahun T. Modeling determinants of time-to-death in premature infants admitted to neonatal intensive care unit in Jimma university specialized hospital. Ann Data Sci. 2017;4(3):361–381. doi: 10.1007/s40745-017-0107-2 [DOI] [Google Scholar]
- 33.Basiri B, Ashari FE, Shokouhi M, Sabzehei MK. Neonatal mortality and its main determinants in premature infants hospitalized in neonatal intensive care unit in Fatemieh Hospital, Hamadan, Iran. J Compr Pediatr. 2015;6(3):e26965. doi: 10.17795/ijcp-26965 [DOI] [Google Scholar]
- 34.Lyoke AC, Lawani OL, Ezugwu EC, et al. Prevalence and perinatal mortality associated with preterm births in a tertiary medical center in South East Nigeria. Int J Women’s Health. 2014;6:881–888. doi: 10.2147/IJWH.S72229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdallah Y, Namiiro F, Mugalu J, et al. Is facility based neonatal care in low resource setting keeping pace? A glance at Uganda’s National Referral Hospital. Afr Health Sci. 2016;16(2):347–355. doi: 10.4314/ahs.v16i2.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel A, Kandasamy Y. Outcome of premature neonates born in a tertiary neonatal intensive care unit in Nairobi, Kenya. J Pediatr Neonatal Individual Med. 2017;6(1):e060113. doi: 10.7363/060113 [DOI] [Google Scholar]
- 37.de Castro ECM, Leite MÁJ, Guinsburg R. Mortality in the first 24h of very low birth weight preterm infants in the Northeast of Brazil. Rev Paul Pediatr. 2016;34(1):106–113. doi: 10.1016/j.rppede.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sankar MJ, Natarajan CK, Das RR, Agarwal R, Chandrasekaran A, Paul VK. When do newborns die? A systematic review of timing of overall and cause-specific neonatal deaths in developing countries. J Perinatol. 2016;36:S1–S11. doi: 10.1038/jp.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mbawala GB, Fredrick F, Kamugisha E, et al. Factors associated with mortality among premature babies admitted at Bugando Medical Centre, Mwanza - Tanzania. East Afr J Public Health. 2014;11(1):641–645. [Google Scholar]
- 40.Arunda M, Emmelin A, Asamoah BO. Effectiveness of antenatal care services in reducing neonatal mortality in Kenya: analysis of national survey data. Glob Health Action. 2017;10(1):1328796. doi: 10.1080/16549716.2017.1328796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doku DT, Neupane S. Antenatal care and neonatal outcome survival analysis of the association between antenatal care attendance and neonatal mortality in 57 low- and middle-income countries. Int J Epidemiol. 2017;46(5):1668–1677. doi: 10.1093/ije/dyx125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Razeq NMA, Khader YS, Batieha AM. The incidence, risk factors, and mortality of preterm neonates: a prospective study from Jordan (2012–2013). Turk J Obs Gynecol. 2017;14:28–36. doi: 10.4274/tjod.62582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta R, Talukdar B. Frequency and timing of antenatal care visits and its impact on neonatal mortality in EAG states of India. J Neonatal Biol. 2017;6(3):263. doi: 10.4172/2167-0897.1000263 [DOI] [Google Scholar]
- 44.Garg P, Abdel-Latif ME, Bolisetty S, Bajuk B, Vincent T, Lui K. Perinatal characteristics and outcome of preterm singleton, twin and triplet infants in NSW and the ACT, Australia (1994–2005). Arch Dis Child Fetal Neonatal Ed. 2010;95:F20–F24. doi: 10.1136/adc.2009.157701 [DOI] [PubMed] [Google Scholar]
- 45.Papiernik E, Zeitlin J, Delmas D, et al. Differences in outcome between twins and singletons born very preterm: results from a population-based European cohort. Human Reprod. 2010;25(4):1035–1043. doi: 10.1093/humrep/dep430 [DOI] [PubMed] [Google Scholar]
- 46.Yeo KT, Lee QY, Quek WS, et al. Trends in morbidity and mortality of extremely preterm multiple gestation newborns. Pediatrics. 2015;136(2):263–271. doi: 10.1542/peds.2014-4075 [DOI] [PubMed] [Google Scholar]
- 47.Albasri SF, Shouib GM, Bajouh OS, Nasrat HA, Ahmad E, Algreisi FM. Maternal and neonatal outcomes in twin and triplet gestations in Western Saudi Arabia. Saudi Med J. 2017;38(6):657–661. doi: 10.15537/smj.2017.6.17699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muhe LM, Mcclure EM, Nigussie AK, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob Health. 2019;7(8):e1130–e1138. doi: 10.1016/S2214-109X(19)30220-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu F, Kong X, Duan S, et al. Care practices, morbidity and mortality of preterm neonates in China, 2013 – 2014: a retrospective study. Sci Rep. 2019;9:19863. doi: 10.1038/s41598-019-56101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weldearegay HG, Abrha MW, Hilawe EH, Gebrekidan BA, Medhanyie AA. Quality of neonatal resuscitation in Ethiopia: implications for the survival of neonates. BMC Pediatr. 2020;20:129. doi: 10.1186/s12887-020-02029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bello M, Pius S, Ibrahim BA. Characteristics and predictors of outcome of care of preterm newborns in resource constraints setting, Maiduguri, Northeastern Nigeria. J Clin Neonatol. 2019;8:39–46. doi: 10.4103/jcn.JCN [DOI] [Google Scholar]
- 52.Gibbs L, Tooke L, Harrison MC. Short-term outcomes of inborn v. outborn very-low- birth-weight neonates (< 500 g) in the neonatal nursery at Groote Schuur Hospital, Cape Town, South Africa. S Afr Med J. 2017;107(10):900–903. doi: 10.7196/SAMJ.2017.v107i10.12463 [DOI] [PubMed] [Google Scholar]
- 53.Boghossian NS, Geraci M, Edwards EM, Horbar JD. Sex differences in mortality and morbidity of infants born at less than 30 weeks’ gestation. Pediatrics. 2018;142(6):e20182352. doi: 10.1542/peds.2018-2352 [DOI] [PubMed] [Google Scholar]
- 54.Kent AL, Wright IMR, Abdel-latif ME. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics. 2012;129:124–131. doi: 10.1542/peds.2011-1578 [DOI] [PubMed] [Google Scholar]
- 55.O’Driscoll DN, Mcgovern M, Greene CM, Molloy EJ. Gender disparities in preterm neonatal outcomes. Acta Pædiatrica. 2018;107:1494–1499. doi: 10.1111/apa.14390 [DOI] [PubMed] [Google Scholar]