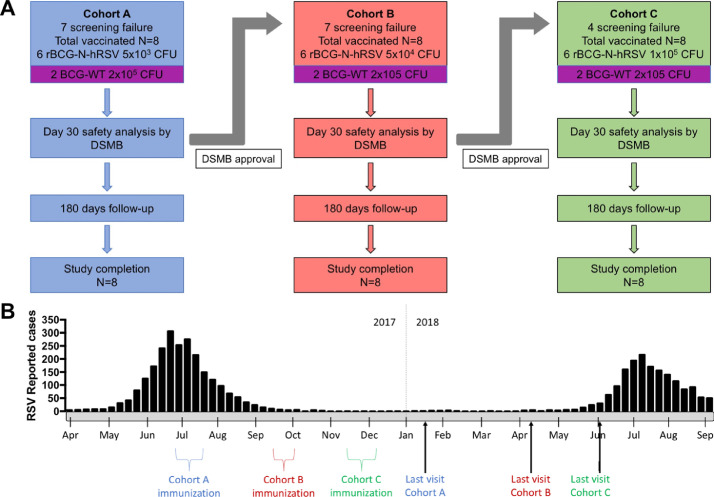
Fig. 1.
Clinical study flow diagram and timeline. This phase 1 clinical study was double-blinded and dose-escalated. (A) Each cohort included 6 volunteers vaccinated with escalating doses of rBCG-N-hRSV (Cohort A: 5 × 103 CFU (blue); Cohort B: 5 × 104 CFU (red); Cohort C: 1 × 105 CFU (green)) and 2 volunteers vaccinated with the standard BCG at full dose (BCG-WT 2 × 105 CFU (purple)). A DSMB evaluated the safety data of each cohort after the first 30 days of follow-up and decided if escalation could continue. (B) A timeline indicating the periods of immunization and end of visits is shown. RSV peaks reported during 2017 and 2018 are also indicated.