Abstract
Purpose
The intravitreal injection (IVI) of pharmacologic agents is the most commonly performed ocular procedure and is associated with a host of complications. Most IVI-related complications data are derived from randomized controlled clinical trials, which report a high adverse event rate. The nature of these protocol-driven trials limit their applicability to the diverse circumstances seen in routine clinical practice. The goal of this study was to determine the prevalence of patient-reported IVI-related complications, their risk factors, and the manner in which patients sought treatment at a tertiary eye care center.
Design
Retrospective, institutional review board–approved study.
Participants
Forty-four thousand seven hundred thirty-four injections in 5318 unique patients at the Cleveland Clinic Cole Eye Institute from 2012 through 2016.
Methods
Intravitreal injection.
Main Outcome Measures
Complication occurrence within 15 days of injection.
Results
From 2012 through 2016, a total of 44734 injections were performed in 5318 unique patients. Overall, complication rates were low, representing 1.9% of all injections, with 1031 unique complications in 685 patients (12.9%). The most common minor complications, or those not requiring intervention, were irritation (n = 312) and subconjunctival hemorrhage (n = 284). The most common serious complications, or those requiring intervention, were corneal abrasion (n = 46) and iritis (n = 31). Most complications (66%) were managed adequately by a telephone or Epic (Epic Systems Corp., Verona, WI) electronic message encounter only. Importantly, no injection protocol parameter, such as type of anesthesia, preparation, or post-injection medication, increased the risk of a complication. However, a patient’s gender, age, number of previous injections, and provider strongly influenced the risk of patient-reported complications.
Conclusions
Overall, complication rates seen in routine clinical practice were low compared with clinical trial reporting. Providers should feel confident in the safety and administration of IVI during times when follow-up office visits and resources may be limited. When performing an IVI, factors such as a patient’s gender, age, number of previous injections, and provider must be taken into account to ensure the best possible outcomes.
Keywords: Complications, Intravitreal injection, Predictive factors, Retrospective
Abbreviations and Acronyms: AMD, age-related macular degeneration; ED, emergency department; IVI, intravitreal injection; RIDE/RISE, Ranibizumab for Diabetic Macular Edema; SCH, subconjunctival hemorrhage; VEGF, vascular endothelial growth factor; VIEW 1/2, VEGF Trap-Eye, Investigation of Efficacy and Safety in Wet AMD
Recent decades have seen a dramatic increase in the number of intravitreal injections (IVIs) performed worldwide.1 The increasing popularity of intravitreal therapeutics among providers has made IVI the most common intraocular procedure, with an estimated 6 million performed annually in the United States as of 2016.1 Although the administration of intravitreal therapeutics often helps to maintain, and even improve, vision, IVI is associated with a host of complications ranging from irritation to infection.
Most data on IVI-related complications are from randomized controlled trials, which are designed with rigorous participant selection criteria, predetermined follow-up times, and other highly controlled conditions aimed at minimizing potential bias. In these protocol-driven trials, adverse event reporting can reach rates as high as 74% to 92% of patients.2 , 3 Although these trials establish internal validity, the applicability of them to diverse patient populations and the variable conditions found in routine clinical practice may be limited.4 For investigators desiring clinically representative data, a well-powered, pragmatic design should be considered.
Although some non-randomized controlled trial studies of IVI-related complications do exist, these are often limited in scope. These types of studies primarily have focused on endophthalmitis, the biggest threat to a patient’s visual acuity. However, endophthalmitis is exceedingly rare, with incidence ranging from 0.015% to 0.08% of injections.5, 6, 7 Other studies examined individual complications such as subconjunctival hemorrhage (SCH) or intraocular inflammation, with reported rates of 10% for SCH8 and 0.09% to 2.9% for intraocular inflammation, depending on the intravitreal agent.9 Furthermore, more recent studies expanded beyond specific complications and reported on post-procedural pain.10 , 11 These studies often were limited to a few agents or a small number of total injections and did not evaluate the collective complications seen in routine clinical practice.
This study aimed to contribute to the paucity of pragmatic research on IVI-related non-infectious complications. We conducted a retrospective cohort study to determine the IVI-related complication rate at a high-volume tertiary eye care center. We then described the initial presentations of these patients to prepare providers better in complication management. Finally, we identified potential risk factors associated with IVI-related complications.
Methods
Identifying Intravitreal Injection–Related Complications
We identified a total of 44 734 injections of 8 common intravitreal agents based on the Current Procedure Terminology code of an intravitreal injection of a pharmacologic agent (67028) at the Cleveland Clinic Cole Eye Institute from 2012 through 2016. These included 4 anti–vascular endothelial growth factor agents—bevacizumab (Genentech, South San Francisco, CA), aflibercept (Regeneron, Tarrytown, NY), and ranibizumab (0.3 mg and 0.5 mg) (Genentech)—and 4 steroidal agents—triamcinolone acetonide (Triesence, Alcon, Fort Worth, TX and Kenalog, Bristol-Myers Squibb, Princeton, NJ), dexamethasone implant (Allergan, Madison, NJ), and fluocinolone acetonide implant (Alimera, Alpharetta, GA). We excluded patients younger than 6 years. To identify IVI-related non-infectious complications, we then screened for patients who underwent an ophthalmology-related encounter within 15 days of the injection date. Each treated eye counted as 1 injection; thus, same-day bilateral injections were counted as 2 separate injections. Our model was adjusted to account for same-day bilateral injections and patients with multiple complications (see “Statistical Analysis”).
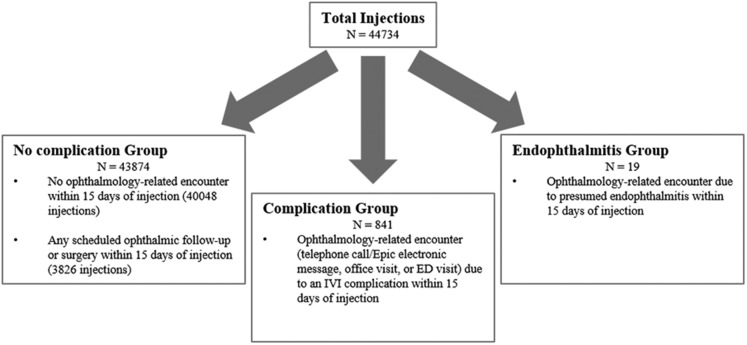
Encounters included telephone call or Epic electronic messages, office visits, or emergency department visits. By manual medical record review, we did not consider any injection that had a scheduled follow-up or scheduled ophthalmic surgery within 15 days after the injection as an IVI-related non-infectious complication. Applying this definition resulted in 841 injections having at least 1 ocular IVI-related complication within 15 days of injection. Criteria for injections categorized in the complication group can be found in Figure 1 . The study was approved by the Cleveland Clinic institutional review board and adhered to the tenets of the Declaration of Helsinki. Informed consent was not obtained due to the retrospective nature of this study.
Figure 1.
Flow chart showing criteria for no complication, complication, and endophthalmitis groups. Patients with endophthalmitis were excluded from this study. ED = emergency department; IVI = intravitreal injection.
Retrospective Record Review of the Sample
For all study participants, we collected baseline demographic and clinical information, including gender, ethnicity, age at injection, eye injected (right, left, or both), intravitreal agent, number of previous injections, and provider. Additionally, we collected the following clinical information for each complication: type of encounter, days to presentation, and type of complication. Complications were divided into 3 groups: minor, serious, and unrelated. Patients with endophthalmitis were excluded from this study; however, we identified 19 presumed cases of endophthalmitis related to an IVI (Fig 1). This observed rate of 0.042%, or 1:2355 injections, was similar to published reports.6 , 12 , 13 Minor complications were those not requiring intervention or observation and included irritation, subconjunctival hemorrhage (SCH), and visual disturbance from an air bubble or medication. Irritation included the following symptoms or pathologic features: discharge, epitheliopathy, keratoconjunctivitis, punctate keratitis, punctate epithelial erosions, redness, superficial keratopathy, or tearing. Serious complications were those requiring either medical intervention or close observation and included complications requiring surgery (e.g., retinal detachment) or intervention (e.g., retinal tear requiring laser). Very rare complications with only 1 occurrence were grouped together under “other” in the serious group. Unrelated complications included those not resulting from the IVI itself, but instead were thought to be the result of disease progression. These were noted based on next available ocular examination chart notes and were confirmed by a trained ophthalmologist.
To identify potential risk factors associated with complications, the injection protocol parameters, including types of anesthesia, preparation, medication used after injection, and anterior chamber paracentesis, were collected (see “Results”). Medications used after injection were those used in the clinic immediately after the injection. These did not include patient prescriptions. These parameters are recorded by our electronic medical record system (Epic).
Statistical Analysis
Descriptive statistics are reported as either count (percent) or median (interquartile range). The primary analysis explored associations among injection protocol factors and any complication. Because each patient received 1 to 110 injections, generalized estimating equation methods were used to account for correlated data (multiple injections per patient) assuming a binomial distribution and a logit link function. An exchangeable correlation structure was assumed in which the observations within a patient are assumed to be equally correlated. Each model included 1 injection protocol factor and adjusted for patient gender, ethnicity, age, whether the injection was a same day bilateral one, provider, and number of visits in sample. When a 0 cell was encountered, it was imputed with a 1 to avoid division by 0. This analysis was repeated for the binary outcomes of any serious complication, irritation, and SCH. A significance level of 0.05 was used. Because this study was exploratory regarding the various steps of the injection protocol, we aimed to maximize the chance of detecting a signal and did not make any adjustments for multiple comparisons. All data analyses were performed using SAS statistical software version 9.4 (SAS Institute, Cary, NC).
Results
The Injected Study Sample
From 2012 through 2016, a total of 44 734 injections were administered in 5318 unique patients at the Cole Eye Institute. Fifty-six percent of the total patient population were women, and 80% were White. Median age at injection was 75.7 years (interquartile range, 65.1–84.2 years). Of the injection characteristics, right-eye and left-eye injections were split evenly, each with 37% of injections, whereas 26% of injections were same-day bilateral injections. Ninety-five percent of injections used an anti–vascular endothelial growth factor agent, with the most common agent being bevacizumab (54%). The median number of injections per patient across the 5-year study period was 5 (range, 1–110). Injections were administered by 16 unique providers with a median number of injections per provider of 2416 (range, 141–6027). Patient and injection characteristics can be found in Table 1 .
Table 1.
Patient (n = 5318) and Injection (n = 44 734) Characteristics of Study Sample
| Characteristic | Data |
|---|---|
| Female gender | 2987 (56) |
| Race or ethnicity | |
| White | 4254 (80) |
| Black | 780 (15) |
| Hispanic | 109 (2) |
| Asian/Pacific Islander | 41 (1) |
| Multiracial | 44 (1) |
| Other | 47 (1) |
| Injection characteristics | |
| Age at injection | 75.7 (65.1–84.2) |
| Right eye | 22 425 (50) |
| Left eye | 22 309 (50) |
| Same-day bilateral injection | 11 638 (26) |
| Agent category | |
| Anti-VEGF | 42 312 (95) |
| Steroid | 2422 (5) |
| Agent | |
| Bevacizumab | 24 376 (54) |
| Aflibercept | 12 433 (28) |
| Ranibizumab (0.3 mg) | 983 (2) |
| Ranibizumab (0.5 mg) | 4520 (10) |
| Triamcinolone acetonide (Triesence) | 1696 (4) |
| Dexamethasone implant | 675 (2) |
| Triamcinolone acetonide (Kenalog) | 39 (< 1) |
| Fluocinolone acetonide implant | 12 (< 1) |
| Year | |
| 2012 | 4369 (10) |
| 2013 | 5640 (12) |
| 2014 | 8891 (20) |
| 2015 | 12 058 (27) |
| 2016 | 13 776 (31) |
VEGF = vascular endothelial growth factor.
Data are presented as no. (%) or median (interquartile range).
Patient Presentation
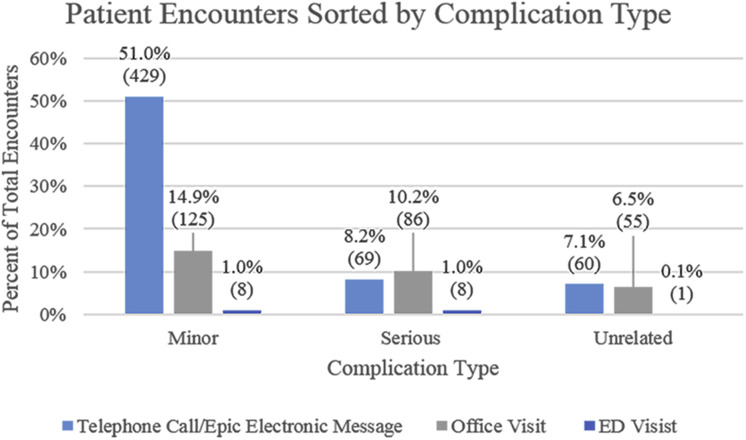
From 2012 through 2016, 1031 unique complications from 685 patients emerged in 841 encounters. Eighty-one percent of these encounters included only 1 complication (Table 2). In 66% of encounters, the patient first sought treatment by telephone call or Epic electronic message. Of these initial telephone call or Epic electronic message encounters, 63.4% were handled by call or Epic electronic message only, 29.2% resulted in a scheduled follow-up visit, and 7.4% resulted in the patient initiating an unscheduled urgent visit after the initial contact. This last patient subset included those who first sought treatment by telephone call or Epic electronic message and were told to “return as needed.” These patients then initiated an unscheduled urgent office visit. This patient subset represented 4.8% of total encounters and sought treatment for only 5.5% of all complications, most of which were minor (Table 3). Office visits and emergency department visits accounted for 32% and 2% of encounters, respectively. Most minor complications first resulted in a telephone call or Epic electronic message, whereas a larger percentage of serious complications first resulted in an office visit compared with a telephone call or Epic electronic message (Fig 2 ). The median number of days to an encounter was 2 days (interquartile range, 1–6 days).
Table 2.
Number (%) of Complications by Encounter Type
| Encounter Type | No. of Complications |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Telephone call/Epic electronic message | 456 (54.2) | 89 (10.6) | 11 (1.3) | 2 (0.2) |
| Office visit | 211 (25.1) | 44 (5.2) | 10 (1.2) | 1 (0.1) |
| ED visit | 12 (1.4) | 4 (0.5) | 1 (0.1) | 0 (0.0) |
| Total | 679 (80.7) | 137 (16.3) | 22 (2.6) | 3 (0.4) |
ED = emergency department.
Data are no. (%).
Table 3.
Complications Reported by Telephone Call or Epic Electronic Message and Urgent Visit Subset
| Complication Type | % of Total Complications (No.) |
|---|---|
| Minor | 3.6% (37) |
| Serious | 1.3% (13) |
| Iritis | 0.3% (3) |
| Corneal abrasion | 0.1% (1) |
| Conjunctivitis | 0.1% (1) |
| Increased intraocular pressure | 0.1% (1) |
| Posterior vitreous detachment | 0.1% (1) |
| Vitreous cell | 0.1% (1) |
| Vitreous hemorrhage | 0.1% (1) |
| Other | 0.4% (4) |
| Unrelated | 0.7% (7) |
| Total | 5.5% (57) |
This subset of the patient population represents those who first sought treatment by telephone call or Epic electronic message and were told by a provider to “return as needed,” after which the patient initiated an unscheduled, urgent office visit. Total complications in all encounters was 1031.
Figure 2.
Bar graph showing patient encounters sorted by complication type. Encounters with multiple complications were categorized by the higher-acuity complication. The total number of encounters was 841. ED = emergency department.
Overall, the rate of any complication was 1.9% of total injections (Table 4). Reported in terms of unique patients, 12.9% of injected patients experienced at least 1 complication. Minor complications were the most common, seen in 1.4% of all injections. Irritation and subconjunctival hemorrhage represented most complications. Serious complications were seen in 0.4% of total injections, with corneal abrasion as the most common. Finally, unrelated complications were noted in 0.3% of total injections (Table 4). No single protocol parameter was significantly associated with any complication (Table 5). The small protocol parameter effects were insignificant when compared with the large effects of patient gender, number of injections performed, and provider. The odds of experiencing any complication increased for women, decreased with each additional injection, and varied greatly among different providers. Provider complication rates ranged from 1.00% to 5.67%, with most providers showing a rate of less than 2.0%.
Table 4.
Complications Observed after Intravitreal Injection
| Complication Type | No. of Total Injections (%) |
|---|---|
| Any complication | 841 (1.9) |
| Any minor complication | 612 (1.4) |
| Irritation | 312 |
| Subconjunctival hemorrhage | 284 |
| Air bubble | 79 |
| Medication in eye | 40 |
| Any serious complication | 157 (0.4) |
| Corneal abrasion | 46 |
| Iritis | 31 |
| Vitreous hemorrhage | 24 |
| Increased intraocular pressure | 22 |
| Posterior vitreous detachment | 12 |
| Conjunctivitis | 11 |
| Hyphema | 6 |
| Vitreous cell | 5 |
| Conjunctival abrasion | 5 |
| Medication in anterior chamber | 4 |
| Corneal edema | 3 |
| Retinal tear | 2 |
| Rhegmatogenous retinal detachment | 2 |
| Other∗ | 19 |
| Any unrelated complication | 124 (0.3) |
| No acute pathologic features | 80 |
| Progressing disease | 44 |
Complications totaled 1031 in 841 encounters, because a patient could demonstrate multiple complications.
Includes one of each of the following: central artery occlusion, complication diagnosed by outside provider, conjunctival inclusion cyst, debris in vitreous cavity, decreased vision, disc edema, episcleritis, exudative retinal detachment, headache, limbal flush, lower eyelid abnormality, macular hole, periorbital ecchymosis, preseptal cellulitis, retained cilia, scleritis, subhyaloid or intraretinal hemorrhage, vitreomacular traction, and vitreous opacity.
Table 5.
Associations among Injection Protocol Parameters and Any Complication
| Injection Protocol Parameter | Present? | No. of Injections | Any Complication, No. (%) | Adjusted P Value∗ |
|---|---|---|---|---|
| Anesthesia | 42 273 | |||
| Subconjunctival injection | Yes | 18 288 | 406 (2.2) | 0.79 |
| No | 23 985 | 416 (1.7) | ||
| Cotton swabs | Yes | 25 478 | 521 (2.0) | 0.24 |
| No | 16 795 | 301 (1.8) | ||
| Topical drops | Yes | 34 265 | 652 (1.9) | 0.93 |
| No | 8008 | 170 (2.1) | ||
| Lidocaine gel | Yes | 9670 | 132 (1.4) | 0.12 |
| No | 32 603 | 690 (2.1) | ||
| Retrobulbar injection | Yes | 11 | 1 (9.1) | 0.40 |
| No | 42 262 | 821 (1.9) | ||
| Preparation | 42 027 | |||
| 10% povidone–iodine | Yes | 24 639 | 484 (2.0) | 0.37 |
| No | 17 388 | 336 (1.9) | ||
| 5% Betadine | Yes | 25 597 | 467 (1.8) | 0.53 |
| No | 16 430 | 353 (2.2) | ||
| Gentamicin, ciprofloxacin, or ciloxan | Yes | 72 | 0 (0.0) | 0.73 |
| No | 41 955 | 820 (2.0) | ||
| Baby shampoo, eyelid wipes, or both | Yes | 10 | 1 (10.0) | 0.38 |
| No | 42 017 | 819 (2.0) | ||
| None/declined | Yes | 51 | 0 (0.0) | 0.71 |
| No | 41 976 | 820 (2.0) | ||
| Medication after injection | 42 114 | |||
| Antibiotic drops† | Yes | 12 831 | 282 (2.2) | 0.14 |
| No | 29 283 | 533 (1.8) | ||
| Diclofenac | Yes | 3882 | 47 (1.2) | 0.39 |
| No | 38 232 | 768 (2.0) | ||
| 5% Betadine | Yes | 9156 | 142 (1.6) | 0.91 |
| No | 32 958 | 673 (2.0) | ||
| None/declined | Yes | 18 334 | 391 (2.1) | 0.45 |
| No | 23 780 | 424 (1.8) | ||
| Paracentesis | Yes | 555 | 17 (3.1) | 0.25 |
| No | 40 800 | 781 (1.9) |
Adjusted for patient gender, ethnicity, age, whether same-day bilateral injection, provider, and number of visits in sample (frequency).
Gentamicin, ciprofloxacin, gatifloxacin, moxifloxacin, ciloxan, ofloxacin, polymixin, polymixin plus trimethoprim, neomycin-polymyxin-dexamethasone, bacitracin-polymyxin, and tobramycin.
Factors Associated with Specific Complications
We took a closer look at complications that were represented by higher frequencies, allowing us to perform a more focused investigation. These included irritation (n = 312), subconjunctival hemorrhage (n = 284), and any serious complication (n = 157). After adjusting for confounders, regression analysis showed that most protocol parameters were not associated with irritation or SCH. Receiving a medication after injection decreased the risk of irritation (Table S1, available at www.ophthalmologyretina.org; P = 0.045), whereas the use of a subconjunctival injection of lidocaine increased the risk of SCH (Table S2, available at www.ophthalmologyretina.org; P = 0.005). Medications received in the clinic after injection included antibiotic drops (e.g., gentamicin, ciprofloxacin, gatifloxacin, moxifloxacin, ofloxacin, polymyxin, polymyxin plus trimethoprim, neomycin-polymyxin-dexamethasone, bacitracin-polymyxin, and tobramycin), diclofenac drops, or 5% povidine-iodine. Again, patient gender, number of previous injections, and provider showed a statistically significant effect across all protocol parameters (data not shown). Consistently, the risks of irritation and SCH increased for women, whereas these risks decreased with each additional injection. For both complications, significant variability in rates among providers remained.
Serious Complications
When modeling the outcome of any serious complication, no associations were found with injection protocol parameters. Interestingly, the gender of the patient no longer impacted risk, but rather the patient’s age at injection played a role. Like previous analyses, the number of previous injections remained a statistically significant factor. Consistently across all protocol parameters, as the patient’s age increased, the risk of any serious complication decreased; furthermore, as the number of injections increased, the risk decreased. The provider no longer impacted the risk of any serious complication.
Discussion
With this retrospective review, we characterize common complications seen in routine clinical practice after IVI. Our analysis showed a complication rate of 1.9% of all injections, or 12.9% of all patients. This rate was significantly less than clinical trial adverse event reporting, such as those in the Ranibizumab for Diabetic Macular Edema (RISE, RIDE), and VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD (VIEW 1, VIEW 2) trials, which showed rates of 90.1%, 81.2%, 76%, and 62.5% of patients, respectively.2 , 3 These protocol-driven studies, in the quest to establish safety and efficacy, use pre-determined follow-up examinations at which all adverse events were reported. These studies reported complication rates observed by investigators, whereas our study looked at patient-initiated encounters concerning a complication, which more closely represents what is encountered in routine clinical practice. Unlike in a clinical trial, patients may not always report or even notice a complication, explaining the difference between the rates observed in clinical trials and the present study.
The overall low rate of complications suggests a low burden on additional health care resources after IVI. Most complications were handled adequately by a telephone or Epic electronic message encounter without a follow-up office visit, because most of these complications were minor. This analysis shows that providers were able to identify properly patients needing an office visit. Especially with the current coronavirus crisis, where most retina providers are still performing IVI, or in situations where patients have limited access to medical care or transportation, knowledge of anticipated complication rates, including those that can be handled with a virtual or telephone encounter and those of a more serious nature, is helpful for resource planning.
This study, in contrast to prior reports, included more types of serious complications and a larger number of injections, lending its application to the general population.14, 15, 16, 17 The overall low complication rate and even lower serious complication rate should provide confidence to patients and providers when discussing the safety profile of this common intervention. Providers also should be aware that most non-infectious complications emerge within 2 days of injection, whereas other studies have shown that most infections emerge after 2 days.18
Interestingly, this study did not uncover any specific injection protocol parameters that significantly increased the risk of any complication developing. Looking specifically at SCH and irritation, subconjunctival anesthesia increased the risk of SCH. More surprisingly, use of medicated eye drops after the injection decreased the risk of irritation. The effect is small and, although statistically significant, may have less clinical importance. However, perhaps the use of drops in the office provided a lubricating effect or flushed out residual povidine-iodine that had been administered in preparation for the injection. A tendency to rinse the ocular surface more after using a disinfectant drop at the end of the procedure may have occurred that also may ease irritation after the injection. It is also important to note that all providers in this study recommend the use of artificial tears on an as-needed basis after injections. The use of artificial tears by the patient could have an impact on subsequent complication presentations; however, this parameter was not captured by our electronic medical record system, and thus was not included in our analysis.
Our study suggests that a patient’s gender, number of previous injections, and provider strongly impact the risk of any complication. Women were at a 32% increased risk of complications compared with men. This could point to gender differences in the use of health care services.19 Psychological factors also could come into play with social constructs such as masculinity.20 , 21 The exact reason still needs investigation, but providers should be aware that men may not seek treatment for complications as frequently as women. With serious complications, age at injection, rather than gender, played a role. Changes in ocular anatomic or physiologic features could result in these differences.22 Younger patients may have a more robust inflammatory system that could account for the higher rates of inflammation after injection.23 Other explanations include older patients receiving more injections, and therefore such patients being more familiar with, and less concerned with, disturbances after the injection or being less capable of initiating a complication encounter. To this latter point, serious complications among older patients could be underreported because of obstacles arranging transportation to appointments or technological barriers in scheduling appointments or calling their eye care center. This underreporting may encourage providers to assess their older patients’ access to care and follow-up after injection.
Surprisingly, our analysis showed that as the number of previous injections increased, the risk of a complication decreased. One possible explanation is that as the patient received more injections, reinforcement of expectations by the patient or counseling by the provider improved when repeated, thus familiarizing the patient with complications after the procedure. This familiarity may decrease their propensity to initiate a complication encounter. Another possible explanation is that patients experiencing a complication simply stopped receiving injections, whereas those not experiencing a complication continued to undergo the procedure. This explanation seems less likely, however, because patients still would need treatment for their underlying eye disease.
In our analysis, complication rates varied among providers. This suggests that subtle differences specific to each provider and not reported in the electronic medical record system may be factors. These differences may include differences in time spent with the patient, the use of a lid speculum, duration of eye rinse after injection, or other factors. The number of total injections performed by each provider also varied, which may play a role in the different complication rates. A provider who focuses discussion on anticipation of the most common complications may lead to fewer complication presentations. Unlike previous studies that relied on provider surveys to associate injection protocol parameters with complications, our study relied on data reported in our electronic medical record system.12 It is interesting to note that the provider has a considerable influence on complications, and we caution the sole use of provider surveys to document injection protocol parameters.
Limitations of our study include the possible underreporting of complications resulting from its retrospective nature and self-reporting. Furthermore, we are unable to standardize the instructions for after injections given by providers, and different extents of education on symptoms after injection may influence patient encounters. A scenario in which patients receive an injection within our health system, experience a complication, and then follow up at a location outside the system is a possibility, albeit one likely to represent only a very small fraction of visits. Finally, with any retrospective study, some aspects could not be captured, such as lid speculum use1 , 24 and injection site,1 , 25 that may or may not play a role in complications. Missing data also limited the study, but the size of our study should help mitigate any effects on rates.
Overall, this study described the presentation of common, non-infectious IVI-related complications and factors associated with the risks of complications. Complication rates seen in routine clinical practice are low: approximately 1.9% of all injections. Serious complication rates overall are very low, and most complications can be handled adequately by telephone or Epic electronic message. Factors influencing the likelihood of patient-reported complications include gender, age at injection, number of previous injections, and provider and must be weighed during the follow-up period. Through this analysis of IVI-related complications, we hope to prepare providers better to achieve the best possible outcomes for their patients.
Manuscript no. ORET-2020-585.
Footnotes
Supplemental material available at www.ophthalmologyretina.org.
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): J.P.E.: Financial support – Aerpio, Alcon, Allergan, Genentech, Novartis, Regeneron, Oxurion; Consultant – Aerpio, Alcon, Allegro, Allergan, Genentech/Roche, Leica, Novartis, Regeneron, Santen, Oxurion, Zeiss; Patent – Leica, P.K.K.: Consultant – Alcon, Allegro, Allergan, Bayer, Boehringer Ingelheim, Kodiak, Novartis, Oxurion, Regeneron, R.P.S.: Financial support – Apellis, Aerie, Graybug; Consultant - Alcon, Genentech, Novartis, Regeneron, Zeiss, A.V.R.: Financial support – Genentech; Consultant – Alcon, Allergan, Novartis, Zeiss; S.K.S.: Financial support – Allergan, Gilead, Regeneron; Consultant – Bausch and Lomb, Clearside, Eyepoint, Novartis, Regeneron, RegenexBio, Santen, Zeiss; Patent – Leica, A.S.B.: Financial support – Regeneron; Consultant – Genentech; Sumit Sharma: Consultant – Alimera, Allergan, Bausch and Lomb, Clearside, Eyepoint, Genentech, Regeneron
Supported in part by the Heed Ophthalmic Foundation (San Francisco, CA) (awarded to S.A.).
Andrew P. Schachat, the Editor-in-Chief of this journal, was recused from the peer-review process of this article and had no access to information regarding its peer-review.
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committee at Cleveland Clinic approved the study and determined that informed consent was not required for this study. All research adhered to the tenets of the Declaration of Helsinki.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Ramos, Xu, Yuan, Nowacki
Analysis and interpretation: Ramos, Xu, Arepalli, Yuan, Nowacki
Data collection: Ramos, Xu, Singuri, Castillo Tafur, Arepalli, Ehlers, Kaiser, R.P.Singh, Rachitskaya, Srivastava, Sears, Schachat, Babiuch, Sharma, Martin, Lowder, A.D.Singh, Yuan
Obtained funding: N/A
Overall responsibility: Ramos, Xu, Singuri, Castillo Tafur, Arepalli, Ehlers, Kaiser, R.P.Singh, Rachitskaya, Srivastava, Sears, Schachat, Babiuch, Sharma, Martin, Lowder, A.D.Singh, Yuan, Nowacki
Supplementary Data
References
- 1.Grzybowski A., Told R., Sacu S., et al. 2018 Update on intravitreal injections: Euretina expert consensus recommendations. Ophthalmologica. 2018;239:181–193. doi: 10.1159/000486145. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen Q.D., Brown D.M., Marcus D.M., et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 3.Heier J.S., Brown D.M., Chong V., et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Patsopoulos N.A. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13:217–224. doi: 10.31887/DCNS.2011.13.2/npatsopoulos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah C.P., Garg S.J., Vander J.F., et al. Outcomes and risk factors associated with endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmology. 2011;118:2028–2034. doi: 10.1016/j.ophtha.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Fileta J.B., Scott I.U., Flynn H.W. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retin. 2014;45:143–149. doi: 10.3928/23258160-20140306-08. [DOI] [PubMed] [Google Scholar]
- 7.Xu K., Chin E.K., Bennett S.R., et al. Endophthalmitis after intravitreal injection of vascular endothelial growth factor inhibitors: management and visual outcomes. Ophthalmology. 2018;125:1279–1286. doi: 10.1016/j.ophtha.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Ladas I.D., Karagiannis D.A., Rouvas A.A., et al. Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: our experience after 2,000 injections. Retina. 2009;29:313–318. doi: 10.1097/IAE.0b013e31819a5f98. [DOI] [PubMed] [Google Scholar]
- 9.Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56:95–113. doi: 10.1016/j.survophthal.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Popovic M.M., Muni R.H., Nichani P., Kertes P.J. Topical nonsteroidal anti-inflammatory drugs for pain resulting from intravitreal injections: a meta-analysis. Ophthalmol Retina. 2020;4:461–470. doi: 10.1016/j.oret.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan R.I., Rosen R.B., Gentile R.C. Optimizing the patient experience and satisfaction: the role of topical NSAIDs with intravitreal injections. Ophthalmol Retina. 2020;4:459–460. doi: 10.1016/j.oret.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Stem M.S., Rao P., Lee I.J., et al. Predictors of endophthalmitis after intravitreal injection: a multivariable analysis based on injection protocol and povidone iodine strength. Ophthalmol Retina. 2019;3:3–7. doi: 10.1016/j.oret.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reibaldi M., Pulvirenti A., Avitabile T., et al. Pooled estimates of incidence of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents with and without topical antibiotic prophylaxis. Retina. 2018;38:1–11. doi: 10.1097/IAE.0000000000001583. [DOI] [PubMed] [Google Scholar]
- 14.Jonas J.B., Spandau U.H., Schlichtenbrede F. Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye. 2008;22:590–591. doi: 10.1038/eye.2008.10. [DOI] [PubMed] [Google Scholar]
- 15.Severn P.S., Hamilton R. The incidence of serious complications associated with intravitreal therapy in a quaternary ARMD service (2008–2014) Eye (Lond) 2015;29(1):150. doi: 10.1038/eye.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozkiris A., Erkilic K. Complications of intravitreal injection of triamcinolone acetonide. Can J Ophthalmol. 2005;40:63–68. doi: 10.1016/S0008-4182(05)80119-X. [DOI] [PubMed] [Google Scholar]
- 17.Bayar S.A., Altinors D.D., Kucukerdonmez C., Akova Y.A. Severe corneal changes following intravitreal injection of bevacizumab. Ocul Immunol Inflamm. 2010;18:268–274. doi: 10.3109/09273948.2010.490630. [DOI] [PubMed] [Google Scholar]
- 18.Mezad-Koursh D., Goldstein M., Heilwail G., et al. Clinical characteristics of endophthalmitis after an injection of intravitreal antivascular endothelial growth factor. Retina. 2010;30:1051–1057. doi: 10.1097/IAE.0b013e3181cd47ed. [DOI] [PubMed] [Google Scholar]
- 19.Farrimond H. Beyond the caveman: rethinking masculinity in relation to men’s help-seeking. Health (Irvine Calif) 2012;16:208–225. doi: 10.1177/1363459311403943. [DOI] [PubMed] [Google Scholar]
- 20.Addis M.E., Mahalik J.R. Men, masculinity, and the contexts of help seeking. Am Psychol. 2003;58:5–14. doi: 10.1037/0003-066x.58.1.5. [DOI] [PubMed] [Google Scholar]
- 21.Gough B. The psychology of men’s health: maximizing masculine capital. Heal Psychol. 2013;32:1–4. doi: 10.1037/a0030424. [DOI] [PubMed] [Google Scholar]
- 22.Grossniklaus H.E., Nickerson J.M., Edelhauser H.F., et al. Anatomic alterations in aging and age-related diseases of the eye. Invest Ophthalmol Vis Sci. 2013;54:ORSF23–ORSF27. doi: 10.1167/iovs.13-12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aspinall R., Lang P.O. Interventions to restore appropriate immune function in the elderly. Immun Ageing. 2018;15:5. doi: 10.1186/s12979-017-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman D.A., Mason J.O., Emond T., McGwin G. Povidone-iodine contact time and lid speculum use during intravitreal injection. Retina. 2013;33:975–981. doi: 10.1097/IAE.0b013e3182877585. [DOI] [PubMed] [Google Scholar]
- 25.Fagan X.J., Al-Qureshi S. Intravitreal injections: a review of the evidence for best practice. Clin Exp Ophthalmol. 2013;41:500–507. doi: 10.1111/ceo.12026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.