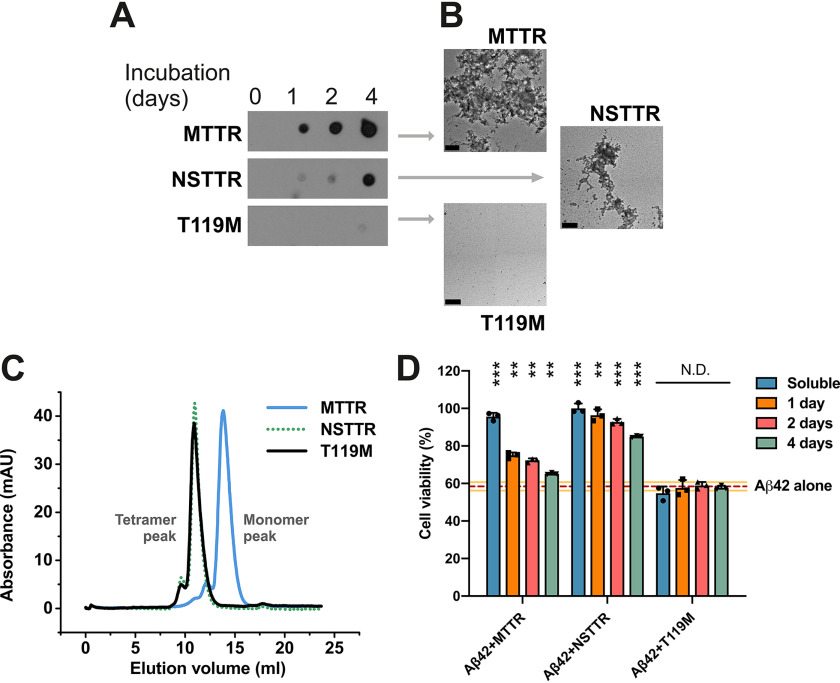
Figure 1.
The inhibition of Aβ42 cytotoxicity by TTR depends on the aggregation propensity of each TTR variant. A, aggregation assay of three TTR variants followed by immunodot blot of insoluble fractions collected prior to incubation or after 1, 2, and 4 days of incubation at 37° C, as labeled. All samples were treated equally and onto the same membrane. Cropping was applied for ethetical reasons. B, electron micrographs of aggregated TTR variants after 4 days of incubation. Scale bar, 200 nm. C, size-exclusion chromatography of soluble TTR variants at pH 7.4. D, cytotoxicity assay of Aβ42 in the presence of TTR variants at different stages of aggregation, followed by MTT reduction. A 5-fold molar excess of soluble TTR (50 μm, considering monomeric concentration) and aggregated samples collected after 1, 2 and 4 days of incubation were added to soluble 10 μm Aβ42 and incubated overnight. The samples were added to HeLa cells, and MTT reduction was measured after 24 h. Buffer-treated cells were considered 100% viability and used for normalization (n = 3). All replicates are shown. Error bars, S.D. **, p ≤ 0.005; ***, p ≤ 0.0005. N.D., significance not detected. These results suggest that the dissociation of the tetrameric TTR structure precedes Aβ42 cytotoxicity protection, whereas aggregated TTR does not exert any effect.