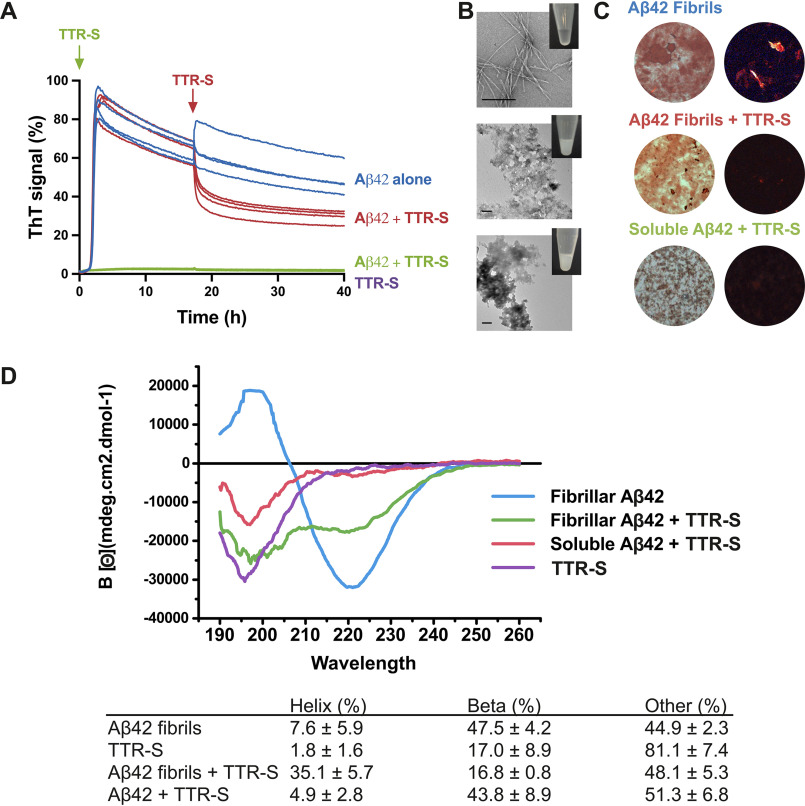
Figure 3.
Effect of TTR-S on both soluble and fibrillar Aβ42. A, ThT fluorescence was measured from samples containing 10 μm Aβ42 in the presence and absence of a 3-fold molar excess of TTR-S. TTR-S was added before incubation (green) or after 18 h of incubation (red). TTR-S alone (purple) and buffer (not shown) were used as negative controls (n = 4). All replicates are shown. For a complete assessment of Aβ42 inhibition by TTR-derived peptides, see Fig. S4. B, electron micrographs of Aβ42 fibrils formed in the absence of inhibitor (top panel), Aβ42 amorphous aggregates formed when TTR-S was added after 18 h of incubation (middle panel), and Aβ42 amorphous aggregates formed when TTR-S was added prior to incubation (bottom). Scale bar, 400 nm. Insets, pictures of test tubes containing the same samples shown by EM but 10 times more concentrated. These pictures were taken after 30 min of incubation and reveal obvious precipitation upon addition of TTR-S. C, Congo red staining of samples shown in A under bright field (left panels) and polarized light (right panels). D, CD traces of Aβ42 fibrils, Aβ42 fibrils after addition of TTR-S, and soluble Aβ42 after addition of TTR-S. The table below shows the calculated percentage of various structural conformations: helix, β-stranded, and others (turns and unstructured). These analyses indicate that the TTR-derived peptide TTR-S inhibits Aβ42 aggregation and promotes the formation of nonamyloid amorphous aggregates.