Scheme 1. Synthetic Route of FAK-PROTACs.
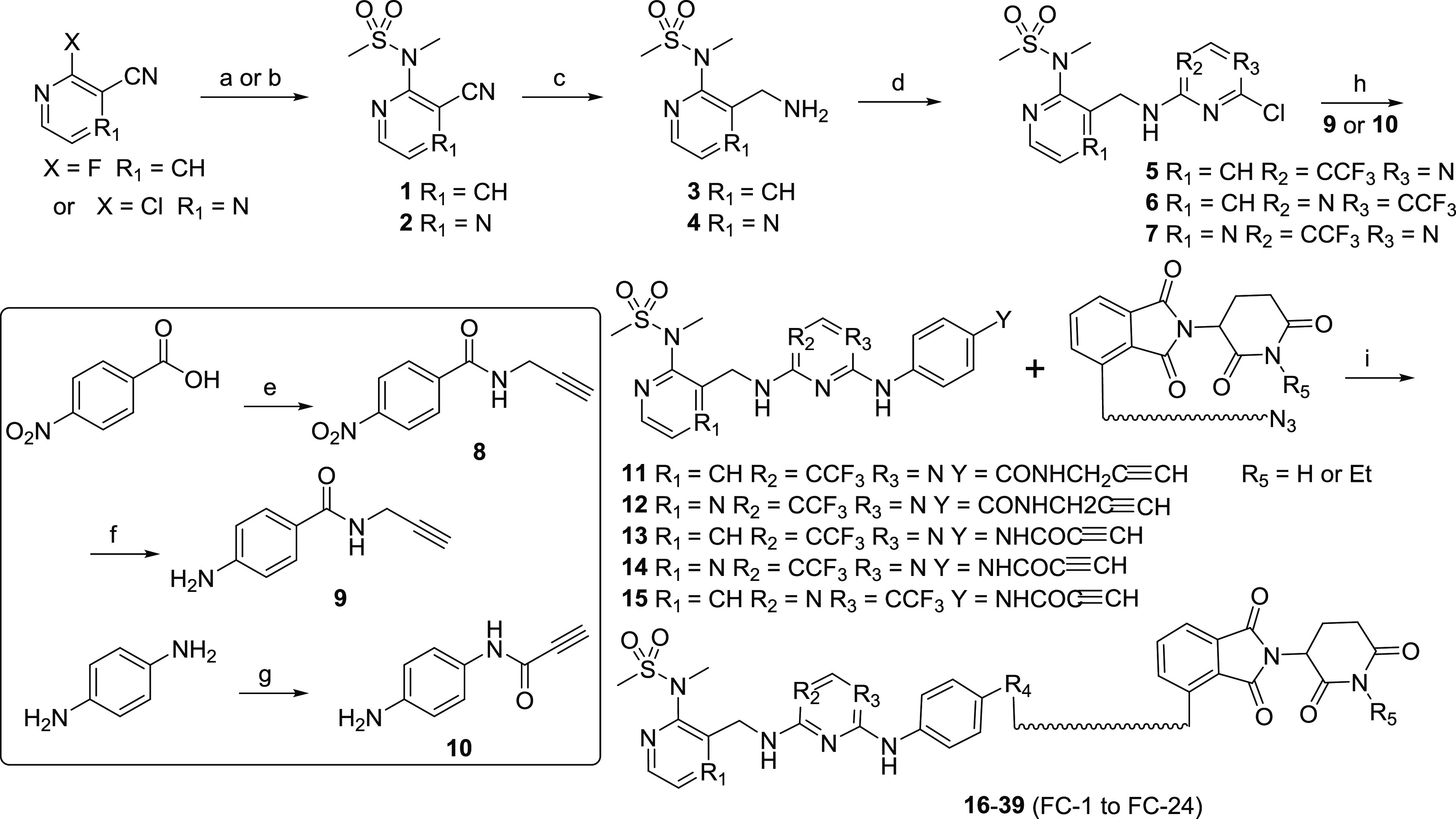
Reaction conditions: (a) t-BuOK, DMF, reflux, 2 h for compound 1; (b) Cs2CO3, MeCN, 70 °C, 20 h for compound 2; (c) Pd/C, H2, EtOH/DMF, rt, 16 h; (d) 2,4-dichloro-5-(trifluoromethyl) pyrimidine, TEA, MeOH, rt, overnight; (e) i: SOCl2, reflux, ii: propargulamine, K2CO3, THF, rt, 16 h; (f) Fe, NH4Cl, EtOH/H2O, reflux, 4 h; (g) propiolic acid, DCC, DMAP, DEE/DMF/CHCl3, rt, 1 h; (h) AcOH, t-amyl alcohol, reflux, 4 h; (i) CuSO4, sodium ascorbate, t-BuOH/H2O, 70 °C, 8 h.
