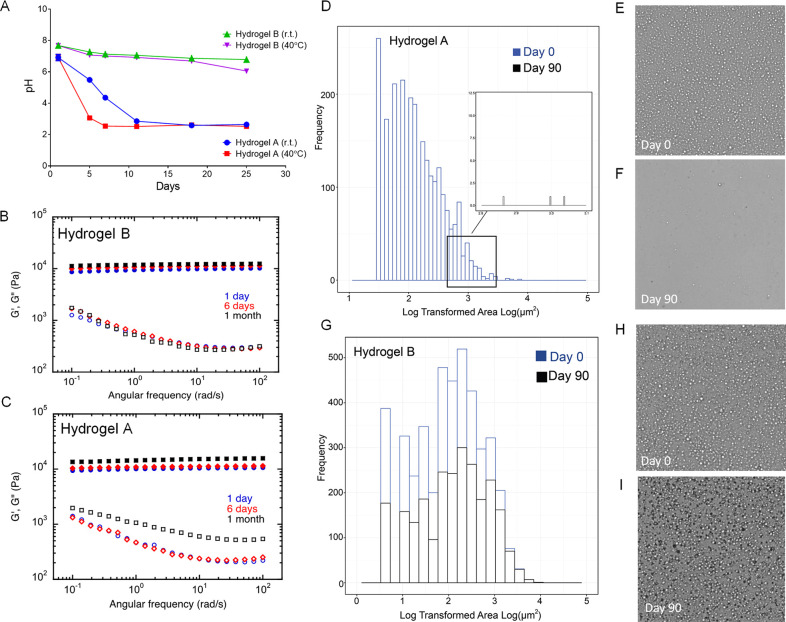
Figure 4.
Impact of buffer on hydrogel stability. (A) pH measurements over time show a sharp drop in pH in nonbuffered cross-linked hydrogel (Hydrogel A) upon storage at room and elevated (40 °C) temperature. pH remains stable over time in the buffered cross-linked hydrogel (Hydrogel B). (B–C) Rheological behavior comparisons between buffered (Panel B) and nonbuffered (Panel C) PFPE/PEI/F127 cross-linked hydrogels. Elastic modulus G′ (filled symbols) and viscous modulus G″ (empty symbols) during oscillatory frequency were measured at three different time-points (day 1, 6, and 30) upon storage at room temperature. Rheological change of the nonbuffered hydrogel indicates physicochemical degradation due to hydrolysis of the PFPE ester end groups. (D–G) Histograms of droplet surface area distributions obtained by cross-polarized optical microscopy of nonbuffered hydrogel A (Panel D) and buffered hydrogel B (Panel G). Microscopy images of hydrogel A (nonbuffered) at day 0 (Panel E) and after 90 days of storage at ambient temperature (Panel F); Microscopy images of hydrogel B (buffered) at day 0 (Panel H) and after 90 days of storage at ambient temperature (Panel I). Droplets degrade over time without the use of buffer, which corresponds to changes in pH (Panel A) and rheological behavior (Panel C).