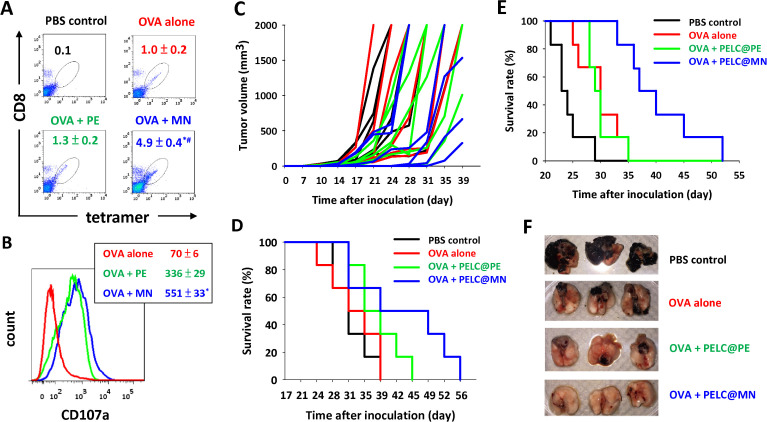
Figure 3.
Intranasal delivery of monodisperse nanoemulsion could generate potent antigen-specific CD8+ cytotoxic T lymphocytes (CTLs) as well as clearance of antigen-transfected cells when combined with tumor-associated antigen therapy. Expansion and activation of antigen-specific CD8+ T cells. (A) The antigen-specific CTL response was determined by SIINFEKL-MHC-I tetramer staining and flow cytometry. (B) The mean fluorescence intensity (MFI) of CD107a on CD8+ T cells. Data are expressed as the mean±SEM from individual murine splenic sample. Intranasal delivery of PELC@MN-adjuvanted OVA inhibits tumor in situ. The mice (n=6) were first subcutaneously inoculated in the flank with EG7 tumor cells (1×105 cells per mouse). Seven days after tumor cell inoculation, the mice in each group were intranasally vaccinated with 100 μg per dose OVA protein without or with either PELC@PE or PELC@MN on days 7, 14 and 21. (C) Tumor volume and (D) survival rate. Intranasal delivery of PELC@MN-adjuvanted OVA inhibits lung metastasis. The mice (n=6) were intravascularly inoculated with B16-F10-OVA cells (5×105 cells per mouse). One week later, the mice were intranasally vaccinated once a week for 3 weeks with OVA (100 μg per dose), alone or adjuvanted with either PELC@PE or PELC@MN. (E) The survival rate was monitored two to three times per week. (F) Lung tissues at day 28 after inoculation of tumor cells. *P<0.05 compared with OVA alone. #P<0.05 compared with the PELC@PE group. OVA, ovalbumin; PBS, phosphate-buffered saline.