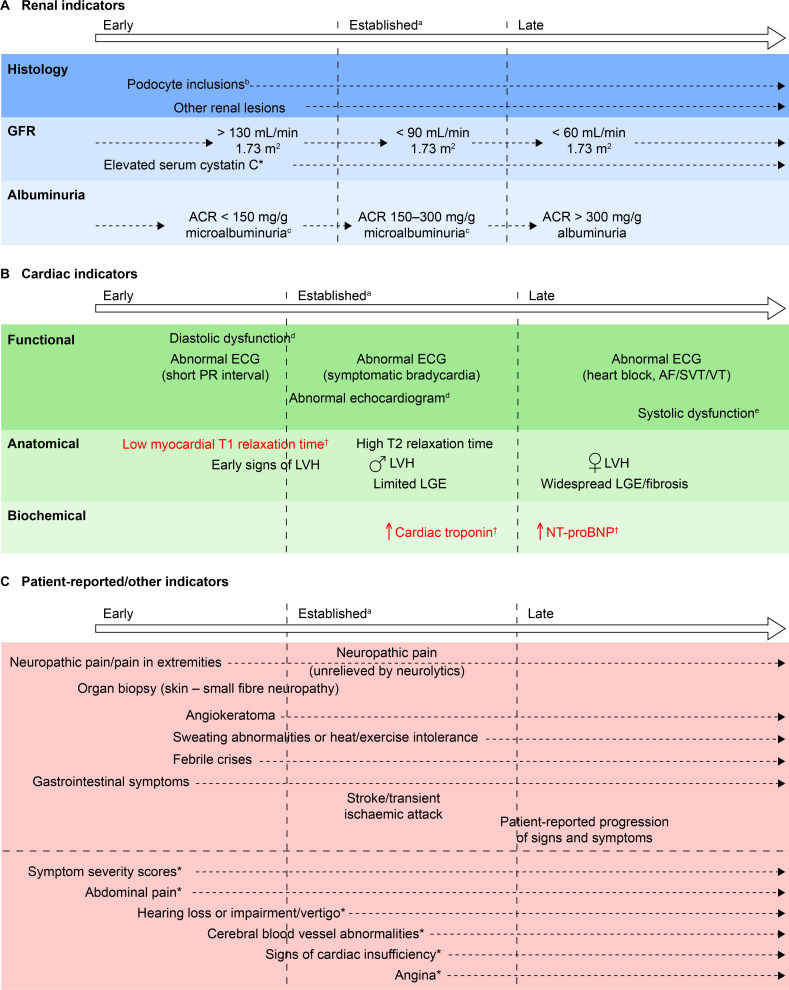
Figure 2.
Chronology of consensus indicators. (A) *Indicator tested for, but not achieving, consensus in round 3. (B) †Indicators in red text achieved consensus both as currently used, and suitable for future adoption, because they are not available in all centres. Two further indicators (abnormal PET/MRI and increased serum lyso Gb3) that were included in round 2 of the initiative but were not taken forward to round 3 are not shown here based on guidance from the cochairs. (C) *Indicator tested for, but not achieving, consensus in round 3. Other indicators tested for, but not achieving, consensus, and which are not included here owing to their lack of specificity were: biomarkers; patient-reported outcomes; absenteeism owing to ill health; and palpitations. aIndicators that currently would be likely to trigger FD-specific treatment initiation. bIn isolation, probably insufficient justification for FD-specific treatment initiation. cMicroalbuminuria could be a trigger for further investigation, such as confirmatory biopsy, and subsequent initiation of disease-specific treatment. dIncluding decreased myocardial strain and strain rate, tissue Doppler abnormalities, enlarged left atrium, abnormal wall motion or pulmonary vein abnormalities. eIncluding shortened PR interval, non-SVT and symptomatic bradycardia. ACR, albumin:creatinine ratio; AF, atrial fibrillation; FD, Fabry disease; GFR, glomerular filtration rate; LGE, late gadolinium enhancement; LVH, left ventricular hypertrophy; lyso Gb3, globotriaosylsphingosine; NT-pro-BNP, N-terminal probrain natriuretic peptide; PET, positron emission tomography; SVT, sustained VT; VT, ventricular tachycardia.