Figure 3.
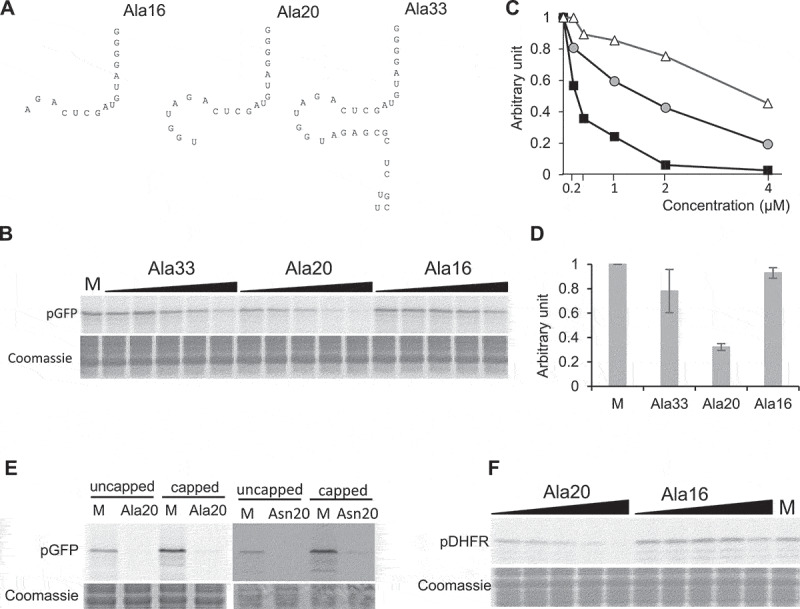
The tRF-5D (Ala) of 20 nt efficiently inhibits translation in vitro. (A) Sequences of the three tRF-5D of 16, 20, and 33 nt (Ala16, Ala20, and Ala33) deriving from Arabidopsis tRNAAla(AGC). (B) Effect of the addition of Ala16, Ala20, and Ala33 on the in vitro synthesis of pGFP protein. The experiment was performed as described in Fig. 1C. Increasing concentrations of synthetic tRFs were used: 0.2, 0.4, 1, 2 and 4 µM. M = Mock, control experiment without tRF. Coomassie blue staining of the gel is shown as a loading control. (C) Relative quantification of in vitro synthesized GFP in the experiment presented in (B). Ala16: white triangles, Ala20: black squares, Ala33: Grey circles. (D) Relative quantification of in vitro synthesized pGFP in experiments similar to that described in (A) and for a tRF concentration of 1 µM. Error bars show standard deviations (mean of three biological replicates). A value of 1 has been given to M. (E) In vitro protein synthesis in a wheat germ translation system of synthesized pGFP transcript in the presence of 35S methionine. Capped or uncapped transcript was used in the absence or presence of Ala20 or Asn20 oligoribonucleotide at 1 µM concentration. M = Mock, control experiment without tRF. Coomassie blue staining of the gel is shown as a loading control. (F) Effect of the addition of Ala16 and Ala20 oligoribonucleotides on the in vitro synthesis of DHFR protein. The experiment was performed as described in Fig. 2B with the same concentration range.
