Figure 1.
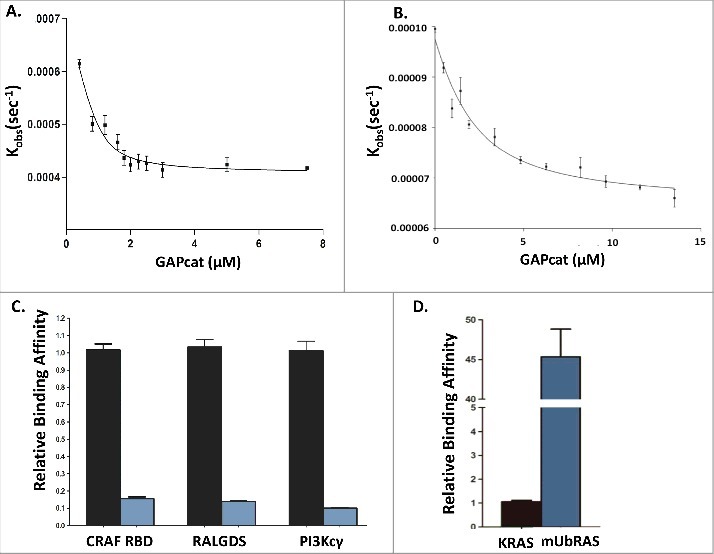
Panel A: Fluorescence binding curve of KRAS (mGppCp) with GAPcat (Kd = 1.9 ± 0.2 μM) compared with Panel B: Fluorescence binding assay of mUbRAS (mGppCp) with GAPcat (Kd = 19.4± 1.4 μM). Panel C: Relative binding affinity of KRAS (mGppCp, black bars) and mUbRAS (mGppCp, blue bars) with effectors CRAF RBD, RALGDS, and PI3Kcγ. Monoubiquitylated KRAS (mGppCp) shows a 7 to 9-decrease in affinity to the CRAF RBD, RalGDS RBD, and PI3Kcγ. Statistical error was determined from 3–5 independent experiments. Panel D: In contrast, mUbRAS (GDP shows 45-fold higher affinity relative to wt KRAS (GDP). Statistical error was determined from 3–5 independent experiments.
