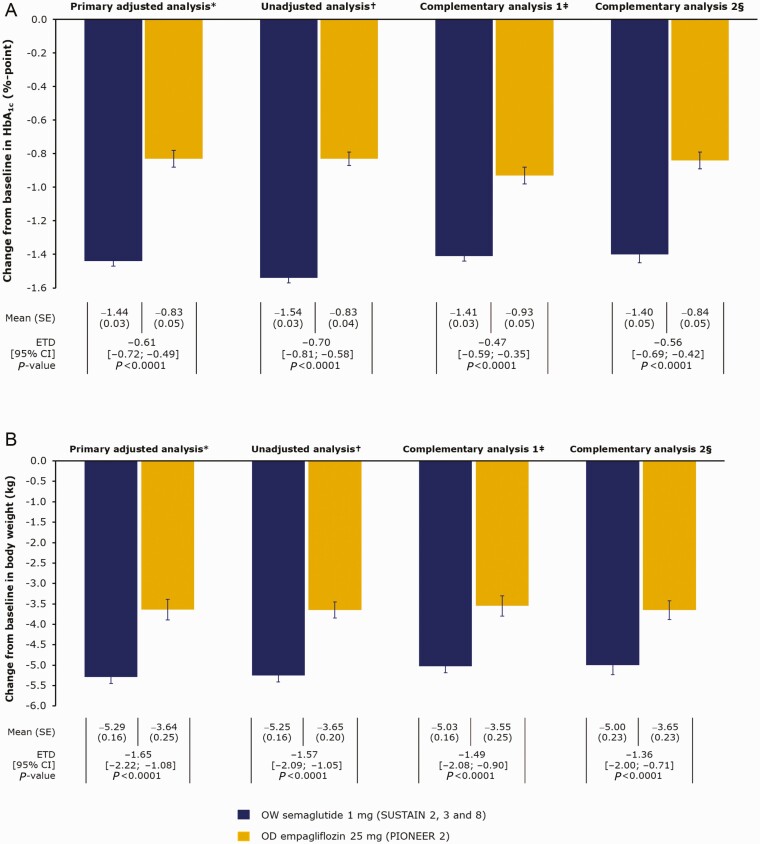
Figure 3.
Change from baseline in a) HbA1c and b) body weight with OW semaglutide 1 mg vs OD empagliflozin 25 mg at week 52. *The primary analysis used data from all randomized patients while on treatment without rescue medication, adjusted for potential prognostic factors and effect modifiers listed in Table 2. †The unadjusted analysis used data from all randomized patients while on treatment without rescue medication, not adjusted for potential prognostic factors and effect modifiers listed in Table 2. ‡Complementary analysis 1 used “in-trial” data (data from all randomized patients irrespective of treatment discontinuation or use of rescue medication). §Complementary analysis 2 included patient data from SUSTAIN 8 and PIONEER 2 only (both 52 weeks in duration; n = 394 for OW semaglutide). Abbreviations: CI, confidence interval; ETD, estimated treatment difference; OD, once-daily; OW, once-weekly; SE, standard error.