Abstract
Aims
The EUropean Comparative Effectiveness Research to Assess the Use of Primary ProphylacTic Implantable Cardioverter-Defibrillators (EU-CERT-ICD), a prospective investigator-initiated, controlled cohort study, was conducted in 44 centres and 15 European countries. It aimed to assess current clinical effectiveness of primary prevention ICD therapy.
Methods and results
We recruited 2327 patients with ischaemic cardiomyopathy (ICM) or dilated cardiomyopathy (DCM) and guideline indications for prophylactic ICD implantation. Primary endpoint was all-cause mortality. Clinical characteristics, medications, resting, and 12-lead Holter electrocardiograms (ECGs) were documented at enrolment baseline. Baseline and follow-up (FU) data from 2247 patients were analysable, 1516 patients before first ICD implantation (ICD group) and 731 patients without ICD serving as controls. Multivariable models and propensity scoring for adjustment were used to compare the two groups for mortality. During mean FU of 2.4 ± 1.1 years, 342 deaths occurred (6.3%/years annualized mortality, 5.6%/years in the ICD group vs. 9.2%/years in controls), favouring ICD treatment [unadjusted hazard ratio (HR) 0.682, 95% confidence interval (CI) 0.537–0.865, P = 0.0016]. Multivariable mortality predictors included age, left ventricular ejection fraction (LVEF), New York Heart Association class <III, and chronic obstructive pulmonary disease. Adjusted mortality associated with ICD vs. control was 27% lower (HR 0.731, 95% CI 0.569–0.938, P = 0.0140). Subgroup analyses indicated no ICD benefit in diabetics (adjusted HR = 0.945, P = 0.7797, P for interaction = 0.0887) or those aged ≥75 years (adjusted HR 1.063, P = 0.8206, P for interaction = 0.0902).
Conclusion
In contemporary ICM/DCM patients (LVEF ≤35%, narrow QRS), primary prophylactic ICD treatment was associated with a 27% lower mortality after adjustment. There appear to be patients with less survival advantage, such as older patients or diabetics.
Keywords: Implantable cardioverter-defibrillator, Risk factors, Mortality, Sudden cardiac death
See page 3448 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa324)
Introduction
Several landmark studies have long established that implantable cardioverter-defibrillator (ICD) therapy improves survival for primary prevention of sudden cardiac death (SCD).1–3 Implantable cardioverter-defibrillators have, therefore, been considered routine treatment since inclusion in international guidelines.4 , 5 Almost two decades later, there is clear evidence that all-cause mortality and appropriate shock rates have decreased6 , 7 and vary considerably dependent on comorbidities.8 Because of these developments, many ICD patients never receive appropriate shocks from their device because of competing risk of non-arrhythmic death9 or because of low arrhythmic risk. The recent results of the DANISH ICD study7 even questioned the overall survival benefit of ICD therapy in patients with non-ischaemic cardiomyopathy (ICM). Improved selection of individual patients with a significant mortality benefit from ICD therapy is urgently required.10 Risk stratification parameters and methods for this purpose are clinically underused.11 , 12 To address this issue, we conducted a large prospective investigator-initiated, non-randomized, controlled, multicentre cohort study at 44 centres across 15 European countries. At the outset and during the conduct of the study, the design of a randomized trial was unanimously considered unethical by the group because of the wide implementation of ICD therapy and unequivocal guidelines. Therefore, as the most meaningful design, we designed a prospective non-randomized controlled study with vigorous statistics and obtained funding from the European Union. We aimed to assess present-day benefit from prophylactic ICD therapy and set out to test multiple combinations of risk factors to predict risks of mortality and ICD shocks vs. risks of non-arrhythmic mortality. Special emphasis was given to identify subgroups with greater or lesser advantage from ICD therapy.
Methods
Study design and patients
The ‘EUropean Comparative Effectiveness Research to assess the use of primary prophylacTic Implantable Cardioverter-Defibrillators (EU-CERT-ICD)’ was funded by the European Community’s Seventh Framework Programme (FP7) as a modular research project to investigate the effectiveness of prophylactic ICDs. The study protocol and the prospective study objectives have been published previously.13 The EU-CERT-ICD prospective trial was an investigator-initiated non-randomized, open, controlled, multicentre cohort study in 2327 patients with ICM or dilated cardiomyopathy (DCM) and candidates for primary prevention ICD therapy by current guidelines.4 Following written informed consent, patients with a minimum age ≥18 years were enrolled if their left ventricular ejection fraction (LVEF) was ≤35% and their New York Heart Association (NYHA) functional class was II or III (or NYHA functional Class I and LVEF ≤30%). Patients were screened from heart failure patients being treated by the local investigators or their institution or referrals for ICD implantation. Based on the large disparities of ICD implant rates between the participating countries,5 we planned to recruit a non-randomized group of 750 comparable patients without ICDs to generate comparative data on current ICD survival benefit. Enrolment to both non-randomized groups occurred simultaneously and consecutively until November 2017, after which date only controls were enrolled to complete the respective group. The decision for or against ICD treatment was not part of the study. Optimal pharmacologic treatment of heart failure and correct timing from the diagnosis of underlying heart disease and acute myocardial infarction was also required. Patients with a secondary prophylactic ICD indication were excluded, as where patients with planned implantation of a device for cardiac resynchronization therapy (CRT-D or CRT-P). Further exclusion criteria were unstable cardiac condition (i.e. acute ischaemia or NYHA IV), persistent higher degree atrioventricular block, previous pacemaker or cardiac device therapy, or a limited life expectancy ≤1 year. In the ICD group, we enrolled more than 1500 analysable patients at first ICD implantation. Participating countries were Hungary, Bulgaria, Croatia, Poland, Slovakia, and the Czech Republic in Eastern Europe; Germany, Belgium, Netherlands, and Switzerland in Western/Central Europe; Denmark, Sweden, Finland in Northern Europe (i.e. Scandinavia); and Spain, and Greece in Southern Europe. Control patients were required to fulfil a primary prevention guideline indication, with reasons for non-ICD status entirely unrelated to the study. It was documented whether the patient refused to have a recommended ICD implanted, whether the ICD was not sufficiently reimbursed by the healthcare system, or whether other reasons existed. After the dropout rate ≈3% was observed, 2330 targeted patients resulted in 2250 analysable patients. The study protocol was approved by all local ethics committees. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) principles.
Outcomes
The primary endpoint was all-cause mortality. Co-primary endpoint in ICD patients was time to first appropriate ICD shock. Secondary endpoints included SCD. All endpoints were reviewed by the external endpoint committee which provided blind adjudication. Each death was classified as SCD, cardiac, non-cardiac, or unknown. ICD shocks were adjudicated after review of device electrograms and classified as appropriate or inappropriate.
Procedures
At enrolment baseline, 12-lead Holter data for 24-h were collected for the purposes of ECG-based risk stratification. Some of these measurements were not possible in atrial fibrillation, therefore, the number of patients with atrial fibrillation was limited to 15% by study protocol. Echocardiography was used to measure LVEF. Underlying cardiac disease, NYHA functional class, pulse rate, resting blood pressure, weight, height, cardiovascular pharmacological treatment, peripheral arterial disease, cerebral vascular disease, pulmonary disease, diabetes mellitus, hypertension, sleep apnoea, tobacco use, any malignant disease, and standard laboratory parameters including creatinine, estimated glomerular filtration rate (eGFR), serum blood urea nitrogen, and N-terminal prohormone of brain natriuretic peptide (NT-pro-BNP) or BNP were documented. All ICD patients were followed in the outpatient clinic or remotely every 3–6 months. By protocol, ventricular tachycardia (VT) and ventricular fibrillation (VF) therapy zones, and a monitor zone were mandated at enrolment. The zone limits actually used at baseline were 173 ± 11 b.p.m., 192 ± 11 b.p.m., and 237 ± 15 b.p.m. During follow-up (FU), 85% (n = 1288) of ICD patients had identical programming, altogether zone limits did not change significantly. Episodes of shock or anti-tachycardic pacing were adjudicated. Patients in the non-ICD control group were scheduled for visits every 6 to 12 months according to their clinical needs. In both groups, information was also retrieved via telephone and/or mail from patients, relatives, general practitioners, hospital records, or local authorities. If patients underwent heart transplant or implantation of a ventricular assist device, FU was censored. Clinical research organization services were provided by the Clinical Trial Unit of the University Medical Center Göttingen. Data quality was monitored in all centres using central monitoring with query management, and additional on-site monitoring. The purpose of monitoring was to ensure optimal data quality and adherence with study protocol and GCP guidelines. Web-based data capture and data collection were done in secuTrial (current version) according to GCP standards.
Statistical analysis
Baseline characteristics are summarized as means (standard deviations) and frequencies (percentages) for continuous and categorical variables, respectively. They are reported overall and by intervention group. The recruiting centres were grouped into four regions as described above.
The primary endpoint all-cause mortality was displayed using Kaplan–Meier curves and analysed using Cox proportional hazards regression. The proportionality assumption was checked by log-minus-log plots of the survival probabilities. To account for the non-randomized nature of this study and the potential differences in patient characteristics between the ICD and control groups, the primary analysis was stratified by region and adjusted for a number of baseline variables which were identified using stepwise selection with P ≤ 0.10 as entry and stay criterion. A number of interactions between the ICD effect and baseline characteristics were considered. These included sex, age group, ICM/DCM, mortality risk group, diabetes, and region. Several propensity score approaches were applied as sensitivity analyses.14 , 15 These included stratification for quintiles of the propensity score, propensity score matching and inclusion of the propensity score as covariate. To build the propensity score a logistic regression analysis was conducted considering a number of potential confounders including demographics, clinical characteristics, such as comorbidities, co-medication, laboratory parameters, and ECG parameters including QRS duration, QT interval duration, and QTc duration as independent variables. When building propensity scores, the description of this well characterized study population included a fairly large number of baseline variables. This also encouraged us to explore a careful model selection as unneccessarily large numbers of covariables should be avoided. Supporting sensitivity analyses were conducted based on a propensity score with model selection from all baseline characteristics mentioned above. The variable selection used stepwise selection with P ≤0.05 as entry and stay criterion. These resulted in confidence intervals (CIs) largely overlapping with those reported in the manuscript based on the procedure with variable selection. To check whether propensity score matching was successful, the so-called Love plots were generated. In the ICD patients, the co-primary endpoint time-to-first appropriate shock was visually summarized by cumulative incidence functions and further analysed by Fine and Gray proportional subdistribution hazard models. Model selection was based on a backward selection procedure with P ≤0.10 as criterion.
All analyses were conducted following the intention-to-treat principle. As the proportion of patients with missing items was 4% (93 out of 2247), complete case analyses were conducted. Two-sided P-values ≤0.05 were considered statistically significant. All analyses were conducted using SAS version 9.4.
As described in the design paper,13 sample size calculations were performed for the comparison of ICD patients with controls regarding mortality and for stratification of the ICD cohort with regard to appropriate shocks and mortality. With exponential survival times, annual all-cause mortalities of 4–5%,12 , 16 a hazard ratio (HR) of 0.7 as observed in MADIT-II,1 recruitment over 3 years, and a study duration of 4 years, a sample of 1500 ICD patients, and 750 control patients was required for a power of 80% at the two-sided significance level of 5%. For the comparison of the primary endpoint between the ICD group and the control group (allocation ratio 2:1), a total number of 279 events was required. From own data,12 we inferred that independent binary or dichotomized risk stratifiers provide HRs of ∼2 between a high- and a low-risk groups. Assuming a group size ratio of 2:1, Schoenfeld’s formula for time-to-event data17 yielded that 122 deaths were required to achieve a power of 95% for a two-sided test at the significance level of 5% assuming an HR of 2. Correspondingly, 108 appropriate ICD shocks were required if the ratio of high and low-risk group sizes was 1:1 and an annual appropriate ICD shock rate of about 4.5% was observed. Assuming exponentially distributed event times, 108 events could be expected to be observed within 4 years as long as at least a total of 1476 ICD patients were recruited. Adjusting for some dropout, we aimed to recruit 1500 patients with ICD.
Results
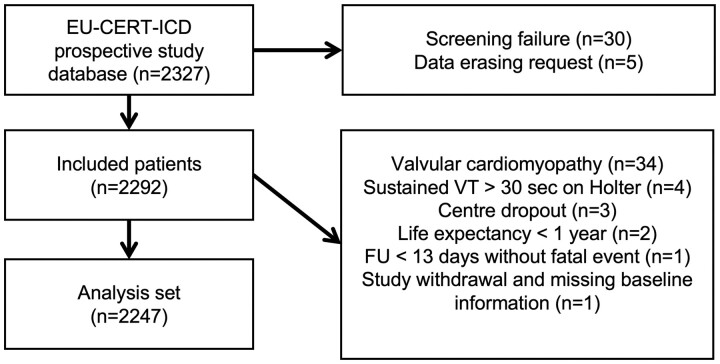
Between 12 May 2014 and 6 September 2018, 2327 patients were enrolled (Figure 1), thereof 1553 ICD patients and 774 controls. Approximately one-half of the patients were from Eastern Europe (n = 1136, 49%). Eastern European countries enrolled a majority of control patients (n = 492, 63%) but only 41% of ICD patients (n = 644). The other regions also contributed significant numbers to both groups, making regional comparisons and stratification by region feasible. After screening failures, data erasing requests, and additional reasons for exclusion from the data set, 2247 patients were analysable. Accordingly, 1516 patients were enrolled at first ICD implantation (ICD group), whereas 731 patients who did not receive an ICD despite an indication were enrolled to the control group. The majority of the control patients (54%) were recruited from countries where primary prophylactic ICD treatment was practically unavailable due to national guidelines or lack of reimbursement (Bulgaria n = 122; Croatia n = 245; Denmark, only for DCM n = 25). In addition, there were 172 patients across all remaining countries noted as ICD refusers, i.e. patients who were offered ICD implantation and had a non-ICD status by personal preference.
Figure 1.
Patient flowchart (n = 2327 patients).
Patient characteristics are shown in Table 1 (for distribution of patient characteristics across regions, see Supplementary material online, Table S1). Despite the non-randomized design, patients in the ICD and control groups were comparable for many but not all variables. For eight variables shown in Table 1, the difference between the ICD and control groups was not significant. For additional variables, the differences were small in absolute and relative terms, however, statistically significant with large patient numbers. Baseline patient characteristics for the ICD vs. control group and stratified by propensity score quintiles are shown in Supplementary material online, Table S2 and standardized differences following propensity score matching are displayed in Supplementary material online, Figure S3. The large majority of patients in both groups received heart failure medications according to the ESC Guidelines. Among others, 94% of all patients had beta blockers, 91% angiotensin converting enzyme or AT1 antagonists, 72% had loop diuretics, and 75% mineralocorticoid receptor antagonists (MRA). The mean LVEF was 28%. The study population was predominantly male, with 18% females in both groups. The mean age was 62 years, 341 patients (15.2%) were aged ≥75 years. As the leading cardiac disease, ICM was diagnosed in 65%. The following devices were implanted at baseline in the ICD group: a single-chamber ICD in 1192 patients (78.6%), a dual-chamber ICD in 298 patients (19.7%), and a subcutaneous ICD (S-ICD) in 24 patients (1.6%). By study protocol, none of the patients was implanted a CRT device. At the decision of the treating physicians, 61 control group patients received an ICD during the study for various reasons (crossover after a mean of 1.1 ± 1.0 years). Extraction of the ICD or deactivation occurred in nine ICD patients (after 0.8 ± 0.8 years). Crossover patients remained in the study on an intention-to-treat basis.
Table 1.
Patient characteristics at baseline (n = 2247)
| Baseline characteristics | ICD group | Control group | Total | Standard difference | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Number of patients | 1516 | 731 | 2247 | |||||
| Female | 274 | (18.1) | 134 | (18.3) | 408 | (18.2) | −0.01 | 0.8822 |
| Region | 0.66 | <0.0001 | ||||||
| Eastern | 644 | (42.5) | 492 | (67.3) | 1136 | (50.6) | ||
| Northern | 150 | (9.9) | 35 | (4.8) | 185 | (8.2) | ||
| Southern | 90 | (5.9) | 78 | (10.7) | 168 | (7.5) | ||
| Western | 632 | (41.7) | 126 | (17.2) | 758 | (33.7) | ||
| Age (years) | 61.9 | 11.5 | 63.4 | 11.7 | 62.4 | 11.6 | −0.13 | 0.0040 |
| BMI (kg/m2) | 27.8 | 5.2 | 28.3 | 5.1 | 27.9 | 5.2 | −0.10 | 0.0050 |
| Creatinine (mg/dL) | 1.156 | 0.589 | 1.225 | 0.600 | 1.179 | 0.594 | −0.12 | <0.0001 |
| Diastolic blood pressure (mmHg) | 74.0 | 11.1 | 75.2 | 11.2 | 74.4 | 11.1 | −0.12 | 0.0061 |
| Haemoglobin (g/dL) | 13.8 | 1.9 | 13.9 | 1.8 | 13.8 | 1.8 | −0.01 | 0.4227 |
| LVEF (%) | 27.5 | 5.6 | 29.1 | 5.5 | 28.0 | 5.6 | −0.30 | <0.0001 |
| QTc (ms) | 438.8 | 38.7 | 431.9 | 51.5 | 436.6 | 43.3 | 0.15 | 0.0015 |
| QRS (ms) | 106.3 | 17.2 | 103.8 | 18.5 | 105.4 | 17.7 | 0.14 | <0.0001 |
| Sodium (mmol/L) | 139.1 | 3.2 | 139.4 | 3.2 | 139.2 | 3.2 | −0.11 | 0.0135 |
| AF (history or present) | 370 | (24.4) | 210 | (28.7) | 580 | (25.8) | −0.10 | 0.0283 |
| COPD | 174 | (11.5) | 76 | (10.4) | 250 | (11.1) | 0.03 | 0.4453 |
| Diabetes | 458 | (30.2) | 223 | (30.5) | 681 | (30.3) | −0.01 | 0.8866 |
| Leading cardiac disease | −0.25 | <0.0001 | ||||||
| Ischaemic cardiomyopathy | 1045 | (68.9) | 416 | (56.9) | 1461 | (65.0) | ||
| Dilated cardiomyopathy | 471 | (31.1) | 315 | (43.1) | 786 | (35.0) | ||
| Malignant disease | 70 | (4.6) | 29 | (4.0) | 99 | (4.4) | 0.03 | 0.4817 |
| NYHA functional class | 0.12 | 0.0067 | ||||||
| Class I or II | 947 | (62.5) | 413 | (56.5) | 1360 | (60.5) | ||
| Class III or IV | 569 | (37.5) | 318 | (43.5) | 887 | (39.5) | ||
| Stroke or TIA | 162 | (10.7) | 61 | (8.3) | 223 | (9.9) | 0.08 | 0.0820 |
| Tobacco use | 976 | (64.4) | 343 | (46.9) | 1319 | (58.7) | 0.36 | <0.0001 |
| Amiodarone | 115 | (7.6) | 111 | (15.2) | 226 | (10.1) | −0.24 | <0.0001 |
| Digitalis glycosides | 100 | (6.6) | 60 | (8.2) | 160 | (7.1) | −0.06 | 0.1640 |
| ACE or AT1 antagonist | 1414 | (93.3) | 635 | (86.9) | 2049 | (91.2) | 0.22 | <0.0001 |
| Beta-blocker | 1436 | (94.7) | 683 | (93.4) | 2119 | (94.3) | 0.05 | 0.2167 |
| Loop diuretic | 1068 | (70.4) | 555 | (75.9) | 1623 | (72.2) | −0.12 | 0.0066 |
| MRA | 1183 | (78.0) | 506 | (69.2) | 1689 | (75.2) | 0.20 | <0.0001 |
Percentages in parentheses. Loop diuretics prescribed were furosemide, torasemide, bumetanide, or piretanide.
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; AT, angiotensin; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
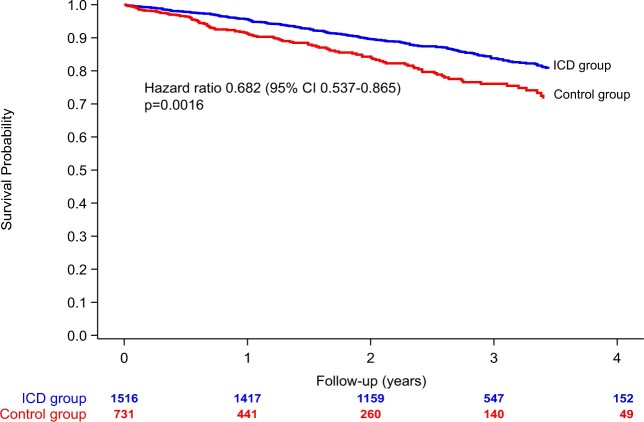
As of 15 May 2019, baseline and FU data (mean 2.4 ± 1.1 years, maximum 4.8 years) of 2247 patients with sample sizes and events as per the initial calculation were analysable in two non-randomized treatment groups. The ICD group had a mean FU time of 2.7 ± 1.0 years compared to 1.7 ± 1.2 years for controls. The respective FU time for ICD shocks was 2.5 ± 1.0 years. During FU, 342 deaths occurred (n = 228 ICD group, n = 114 control, annualized mortalities: 6.3%/years overall, 5.5%/years ICD; 9.2%/years control). Kaplan–Meier curves for mortality are shown in Take home figure. Kaplan–Meier curves for mortality by regions are displayed in Supplementary material online, Figure S4. There was heterogeneity of mortality between regions, largely displayed by a higher all-cause mortality in the Eastern European sites. We, therefore, stratified multivariate models by region. The lowest all-cause mortality was observed in the Scandinavian sites, as compared to western and southern Europe. Supplementary material online, Figure S5 shows that there was no difference in control group survival when comparing patients enrolled during the first half of the study as compared with later patients.
Take home figure.
Unadjusted all-cause mortality of the ICD group (blue line) and the control group (red line). There is an unadjusted 32% difference in survival between the ICD group and the control group. CI, confidence interval.
A stepwise multivariate Cox regression model was used to identify independent predictors of mortality. The final multivariate Cox model is shown in Table 2. There were 93 cases (4.1%) with missing values up to n = 50 (2.2%) for haemoglobin and n = 34 (1.5%) for creatinine. All other missing values were fewer. Multivariable predictors of the primary endpoint included age, LVEF, NYHA class <III, creatinine, chronic obstructive pulmonary disease, and QTc.
Table 2.
Multivariate Cox regression analysis for mortality (final model after variable selection using P < 0.10)
| Parameters | Stratified by region |
|||
|---|---|---|---|---|
| HR | 95% CI | P-value | ||
| Age (per 10 years) | 1.411 | 1.255 | 1.583 | <0.0001 |
| LVEF (per 5%) | 0.762 | 0.688 | 0.841 | <0.0001 |
| QTc (per 40 ms) | 1.322 | 1.173 | 1.431 | <0.0001 |
| COPD (yes vs. no) | 2.191 | 1.691 | 2.837 | <0.0001 |
| BMI (kg/m2) | 0.954 | 0.929 | 0.979 | 0.0004 |
| Haemoglobin (g/dL) | 0.887 | 0.827 | 0.951 | 0.0008 |
| Creatinine (mg/dL) | 1.224 | 1.080 | 1.386 | 0.0015 |
| NYHA (III vs. I–II) | 1.454 | 1.153 | 1.833 | 0.0016 |
| Sex (male vs. female) | 1.580 | 1.152 | 2.166 | 0.0045 |
| Diabetes (yes vs. no) | 1.265 | 0.999 | 1.600 | 0.0506 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
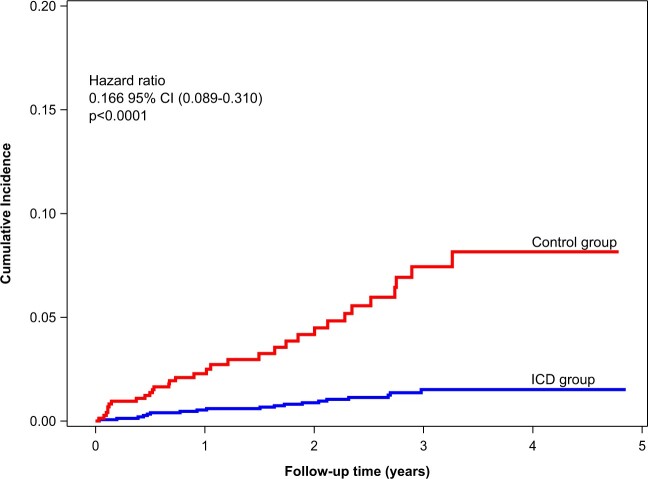
Using the multivariable models for correction, the adjusted difference in survival between the ICD and control group was found to be 27% (adjusted HR 0.731, 95% CI 0.569–0.938, P = 0.0140), see also Supplementary material online, Figure S6. Adjustments based on propensity scoring yielded very similar results. Table 3 shows the results of sensitivity analyses using several adjustment methods to calculate the difference in survival between the ICD and control group. These are consistent with each other. Supplementary material online, Table S7 shows additional sensitivity analyses based on a propensity score with variable selection. Sudden cardiac death was considerably lower in the ICD group as compared to the control group (Figure 2). Nineteen SCDs occurred in the ICD group, while 32 were seen in the control group (unadjusted HR 0.158, 95% CI 0.086–0.293, P < 0.0001). One-hundred and seven patients had a first appropriate shock (annualized rate: 2.8%/year), with a total of 148 appropriate shocks. Of these, 24 patients had two or more appropriate shocks. The VT/VF initially encountered in the first 107 shocks had an average cycle length of 234 ± 37 ms (heart rate 256 b.p.m.), thereof 13 episodes were described as accelerating and the terminated arrhythmia had a cycle length of 199 ± 35 ms (heart rate 302 b.p.m.). The final Fine and Gray competing risk multivariate shock model is shown in Table 4. Only one patient was reported with a successful resuscitation in the control group and was subsequently implanted an ICD. A total of 43 patients (1.9%) were reported with presumed cardiac and potentially arrhythmogenic syncope, 35 in the ICD group (2.3%), and 8 in the non-ICD group (1.1%). A total of 106 inappropriate shocks occurred in 39 patients (annualized rate to first event: 1.0%). ICD revisions during FU occurred in 112 patients (annualized rate: 2.7%). Reasons for reintervention were revisions for lead defects, lead dislocations or perforations n = 52, infection n = 23, pocket haematoma n = 4, generator replacement with or without lead revision n = 8, ICD upgrades n = 13, explantation n = 8, and other n = 4.
Table 3.
Comparison of Cox regression model and propensity score-based models to analyse the difference between treatment groups on survival
| Models | n | Events | HR (ICD vs. control | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Unadjusted strata by region | 2247 | 342 | 0.682 | 0.537 | 0.865 | 0.0016 |
| Adjusted by mortality predictors (primary analysis) | 2154 | 326 | 0.731 | 0.569 | 0.938 | 0.0140 |
| Propensity score as covariate | 2134 | 323 | 0.685 | 0.524 | 0.895 | 0.0056 |
| Strata by propensity score quintiles | 2134 | 323 | 0.691 | 0.532 | 0.897 | 0.0055 |
| Propensity score matching (2:1) | 1460 | 233 | 0.725 | 0.556 | 0.945 | 0.0175 |
Depending on the method used for adjustment of the hazard ratio for ICD vs. control, only small variations between 0.682 and 0.731 in terms of the HR were found (primary analysis: regression model adjusted by mortality predictors; sensitivity analyses: approaches based on propensity scores).
CI, confidence interval; HR, hazard ratio.
Figure 2.
Cumulative incidence of sudden cardiac deaths in the ICD group vs. control group. The incidence of sudden cardiac death is much lower in the ICD group as compared with the control group. .
Table 4.
Multivariate Fine and Gray competing risk regression model for first appropriate shock (final model after variable selection using P < 0.10)
| Parameters | Stratified by region |
|||
|---|---|---|---|---|
| HR | 95% CI | P-value | ||
| Digitalis (yes vs. no) | 2.825 | 1.658 | 4.815 | 0.0001 |
| Sex (male vs. female) | 2.419 | 1.223 | 4.786 | 0.0111 |
| COPD (yes vs. no) | 1.781 | 1.084 | 2.925 | 0.0227 |
| QTc (per 40 ms) | 1.221 | 1.041 | 1.489 | 0.0264 |
| BMI (per kg/m2) | 1.031 | 1.000 | 1.063 | 0.0512 |
| Systolic blood pressure (per 10 mmHg) | 1.116 | 0.990 | 1.255 | 0.0674 |
Out of 1494 included patients, 106 patients experienced at least one appropriate shock, 231 patients experienced competing events, and 1157 patients where censored.
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
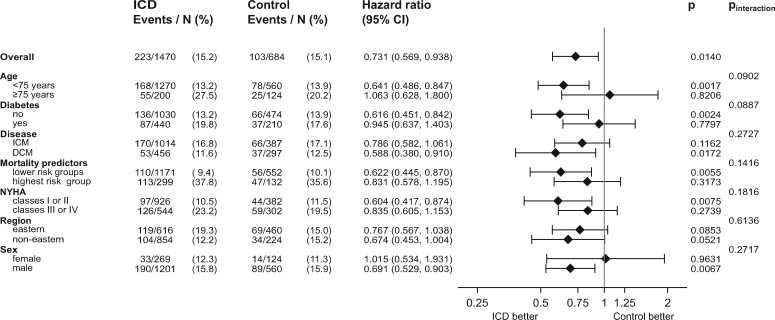
Figure 3 shows that an improved survival associated with the ICD was not present in those aged ≥75 years (adjusted HR 1.063, P = 0.8206, P interaction = 0.0902), or in diabetics (adjusted HR = 0.945, P = 0.7797, P interaction = 0.0887). When stratifying by ICM and DCM, we found lower event rates in DCM patients, but a higher difference between ICD group and control group survival (ICM: HR 0.790, 95% CI 0.580–1.060, P = 0.1160; DCM: HR 0.590, 95% CI 0.380–0.910, P = 0.017, P interaction for ICM/DCM was 0.2727). Patients with NYHA ≥III showed an adjusted HR of 0.835, 95% CI 0.605–1.153, P = 0.2739, while patients with NYHA <III demonstrated a significantly lower mortality associated with the ICD (adjusted HR 0.604, 95% CI 0.417–0.874, P = 0.0075, P interaction = 0.1816). In the eastern European region, we found a 23% lower mortality associated with the ICD (HR 0.770, 95% CI 0.570–1.040, P = 0.0850). The P interaction between regions was 0.6136. In men, the adjusted HR was 0.691, P = 0.0067, in women we found an adjusted HR of 1.015, P = 0.9631, P interaction = 0.2717. In order to test ICD efficacy in relation to underlying mortality risk, the above-described multivariate Cox regression model for mortality was used as a risk score with the cohort divided into risk quintiles both in the ICD group and the control group (Supplementary material online, Figures S8 and S9). In the highest risk quintile of the ICD group, there were 3-year mortalities as high as 40% as well as a smaller difference between ICD and control mortalities (adjusted HR = 0.8880, 95% CI 0.622–1.2680, P = 0.5135). Because of the lower number of events in the low-risk and lowest-risk quintiles, we combined the lower four quintiles for a total of 166 deaths vs. 160 deaths. The adjusted HR for ICD vs. control in the lower four quintiles was 0.6220, 95% CI 0.445–0.870, P = 0.0055. P interaction was 0.142 for the ICD effect in the highest quintiles vs. the lower four quintiles.
Figure 3 .
Forest plot of adjusted hazard ratios, confidence intervals, P-values, and P for interaction for the comparison of mortalities in the ICD group v. the control group. Shown are elderly patients ≥75 vs. <75 years, diabetes vs. no diabetes, ischaemic cardiomyopathy vs. dilated cardiomyopathy as cardiac disease, patients in the highest risk group of mortalities (highest quintile of mortality score) vs. the remaining four quintiles, patients with New York Heart Association ≥III vs. <III, patients from the Eastern region vs. non-Eastern regions, male vs. female patients. The number of patients and events (and the percentage) is given. Note that the duration of follow-up also adds to the difference of hazard ratios. DCM, dilated cardiomyopathy; ICD, implantable cardioverter-defibrillator; ICM, ischaemic cardiomyopathy; NYHA, New York Heart Association functional class.
Discussion
In typical European patients eligible for primary prophylactic ICD therapy, the results of the EU-CERT-ICD study showed that the overall patient cohort exhibited a significant 27% difference between the survivals of the ICD and control groups, representative of ICD survival benefit. This difference was apparent over the whole FU period, and it was reproducible using different statistical methods of adjustment for remaining baseline differences. Matching of baseline characteristics between ICD and control group was overall fairly good, so that residual differences were correctable by appropriate statistical methodologies. To manage the potential differences within Europe, we stratified all analyses by region. Restricting the analyses to Eastern Europe, where the most patients were recruited, demonstrated comparable results and confirmed a 23% ICD survival benefit.
Therefore, our study data confirmed prospectively for prophylactic ICM/DCM patients that the relative benefit found in the early studies MADIT-II, SCD-HeFT and DEFINITE1–3 is sustained 15–20 years later. The usefulness of the ICD for primary prevention of SCD is remarkable in light of the improvement of all-cause mortality and appropriate shock rates in the past decades.6 , 7 , 16 , 18 , 19 However, similar HRs correspond to a similar change in relative risk which may currently be a lower absolute number. The EU-CERT-ICD study is unique in providing contemporary information on outcomes and usefulness of the ICD throughout Europe, as it was conducted in 15 representative countries. The data is not contradicting the more recent DANISH ICD study7 but expands the randomized DANISH data in non-ICM patients to a wider population of ICM and DCM patients with an LVEF ≤35% and potential indications for single- or dual-chamber primary prevention ICD implantation, and without CRT indications. When comparing the ICD survival difference of 27% in our study between ICM and DCM, we found no evidence for a lower benefit in DCM patients. This is in line with the DANISH study because the overall risk was found to be low in Denmark, and a significant proportion of their patients were treated with CRT.
A second finding of our prospective study was that the usefulness of the ICD was unevenly distributed in the overall cohort and was highly reduced in defined patient subgroups. It was our prospective hypothesis to identify such groups,13 to our knowledge EU-CERT-ICD is the first prospective study evaluating these hypotheses. Clinically relevant examples from our data are elderly patients ≥75 years (adjusted HR = 1.063, P = 0.8206, P interaction=0.0902), and diabetics (adjusted HR 0.945, P = 0.7797, P interaction=0.0887). These numbers show that there was a trend towards a significant interaction effect for elderly ≥75 years and diabetics. Of note, our study could not be powered for interaction testing, which would have required a much higher number of patients.20 Borderline P-interactions between 0.05 < P < 0.10 in a study of the present size may therefore not exclude a true effect.
Our findings in diabetics agree well with a meta-analysis of 3345 retrospectively identified patients in the early landmark studies which confirmed that diabetics did not have a survival benefit,21 as well as recent findings from the retrospective EU-CERT-ICD registry project in 3535 patients.22 Importantly, our results concur with a substudy from DANISH23 demonstrating that elderly patients ≥68 years did not experience ICD survival benefit. The overall group of the DANISH study (n = 1116 patients) did not show a significant survival benefit (HR 0.87, 95% CI 0.68–1.12, P = 0.28)7 which we do not consider contradicting our study findings. In the secondary DANISH publication on the effect of age,23 it was shown that younger patients <70 years did benefit from the ICD (HR 0.70, 95% CI 0.51–0.96, P = 0.03) while older patients ≥70 years did not (HR 1.05, 95% CI 0.68–1.62, P = 0.84). A significant interaction of the survival effect for age was found in DANISH (P = 0.009). These effects of age on ICD survival benefit in DANISH are well in line with our current study, where younger patients <75 years did benefit (HR 0.64, 95% CI 0.49–0.85, P = 0.0017) while older patients ≥75 years did not (HR 1.06, 95% CI 0.63–1.80, P = 0.82). We found a trend for a significant interaction of the survival effect for age (P = 0.0902).
Furthermore, for men, our adjusted HR was 0.691, P = 0.0067, with considerable contrast for women the adjusted HR was 1.015, P = 0.9631, P interaction = 0.2717. Notably, only 18% of our patients were women. This would correspond to an absent benefit, as hypothesized in the literature.19 , 24 The interaction-P for these comparisons was non-significant, and higher patient and/or event numbers in this subgroup are needed. This is also true for the ICD patients on the lower end of the mortality spectrum where we could not draw a conclusion from our study because of the low number of events and shorter FU compared to the risk assessment papers from the SCD-HeFT25 and MADIT-II.26 We could confirm findings from SCD-HeFT2 and a later meta-analysis by Friedman et al.27 in that the usefulness of the ICD seems to be better in heart failure patients in NYHA II functional class as compared to NYHA III patients. These examples of absent ICD benefit are best explained with higher competing risks of non-sudden or non-cardiac deaths.9 , 11 , 12 , 25 , 28 Publications comparing ICD vs. non-ICD in typical primary prevention patients have so far relied on meta-analyses of early ICD trials29 or propensity-score matching of selected retrospective registry patients.30
We could demonstrate that a relatively low percentage of patients treated by an appropriate shock per year (2.8%) is associated with a significant difference in overall survival and SCD. We believe that using modern programming as in our study, a large proportion of appropriate shocks are lifesaving. Shocks alone do not fully explain the observed differences, the potential gap might be related to improved awareness and better surveillance of ICD patients. There were moderate regional mortality differences with higher and lower mortality rates in the Eastern European countries and in Scandinavia, respectively. In general, heart failure medications reached very high percentages in all regions and centres of our study. As hypothesized, valid risk scores for mortality and shock could be provided. We confirmed mortality markers identified by multiple study groups including our own with high accuracy.12 , 16 , 25 , 26 , 28 , 31 , 32 In the group with the highest mortality, a comparison with the control group quintile showed that the survival difference associated with the ICD is smaller, a finding similar to Levy et al.25 However, the interaction of the HR in the highest mortality quintile vs. the remaining four quintiles was not significant, P = 0.142. In order to stratify ICD survival benefit further, we have also measured several advanced risk stratification electrocardiographic markers which will be presented separately.13 , 33 , 34 They may also be used in combinations with cardiovascular history and biomarkers, as previously exemplified.11 , 12 Since there were subgroups with no or only a small survival benefit of the ICD, such as older patients, diabetics, or patients with advanced heart failure, individualized treatment strategies need to be explored. While results of randomized studies are needed, these may also include withholding ICD therapy from the described patient subgroups. Since the same subgroups were identified in other studies, controlled randomized investigations now appear justified. Viewed from healthcare payers’ and societies’ perspectives, the decision for or against primary prophylactic ICD implantation bears significant relevance in terms of cost-effectiveness. The EU-CERT-ICD project will subsequently feature health economics analyses incorporating the original study data presented here.13
Based on our study results, primary prophylactic ICD therapy should remain the standard of care in patients with ischaemic or non-ICM and reduced LVEF without CRT treatment. Our study confirmed the overall mortality benefit of prophylactic ICD implantation. No subgroups were identified in which such therapy would be harmful. Nevertheless, the decision for or against ICD therapy should remain case-specific, especially because of its invasiveness and potential complications. Our results, therefore, assist the individual patient, who has to make a personal therapy decision while considering individual circumstances and preferences.
Randomized ICD studies of prophylactic ICD indications are presently ongoing. The RESET-CRT trial (NCT03494933) in patients with an LVEF ≤35% and CRT treatment (CRT-D vs. CRT-P) has been recruiting patients since 2018, and a randomized ICM study has just been funded.35 The I-70 study by the US Veterans Administration (NCT02121158) is randomizing elderly patients ≥70 years to ICD vs. optimal medical therapy. The DO-IT study will report outcomes in 1500 primary prophylactic ICD patients from the Netherlands, however, without control patients.36
Limitations of our study need to be recognized. The study design brought about the limitations and possible biases of a non-randomized, controlled study, including the possibility of different unmeasured or unidentified confounders. Nonetheless, the non-randomized controlled study design was considered as the best feasible design in the planning and funding stage of the project. A randomized design was rejected by the study group when planning the project or later. As demonstrated here, a significant number of patients have a clear benefit from the ICD. The non-randomized study design was accounted for using various advanced statistical methods so that the authors are convinced that the results comparing the two study groups are valid. Because of a somewhat later enrolment during the study, control group FUs were shorter than ICD FUs. There was, however, no difference in non-ICD group survival in control patients enrolled during the first half of the study as compared with later patients. Regular ICD clinic schedules after implantation led to ICD group patients having more frequent visits with treating cardiologists. Missing endpoints based on patient reporting, such as arrhythmogenic syncope or successful resuscitation, cannot be fully ruled out and would have favoured the control group. A treatment bias with improved outcomes in the ICD patients as compared to the control patients cannot be ruled out. However, we do not consider it likely because not all ICD clinics were actively involved in heart failure management. Heart failure treatment in both groups was on a high level, and the statistical methods for adjustment of outcomes specifically reflected medications. Furthermore, only ICD complications leading to revision procedures were reported.
Conclusion
In ICM and DCM patients with an LVEF of 35% or less, narrow QRS and contemporary pharmacological treatment, primary prophylactic ICD treatment showed an adjusted 27% survival benefit in typical European patients with low to moderate mortality. In the ICD group, this benefit was associated with a markedly lower rate of SCD and the percentage of patients treated with appropriate shocks. Since there were patient groups with less ICD survival benefit, such as older patients or diabetics, individualized treatment strategies are needed.
Supplementary Material
Acknowledgements
EU-CERT-ICD Study Investigators (in the order of patients recruited): Department of Cardiology, Semmelweis University Heart Center, Budapest/Hungary: Béla Merkely MD, Peter Perge MD, Zoltan Sallo MD, Gabor Szeplaki MD, Nandor Szegedi MD, and Klaudia Vivien Nagy MD (364 patients); Department of Cardiology and Pneumology, Heart Center, University Medical Center, Göttingen, Germany: Markus Zabel MD, Lars Lüthje MD, Simon Schlögl MD, RajeevaSritharan MSc, Helge Haarmann MD, Leonard Bergau MD, Joachim Seegers MD, Gerd Hasenfuß MD, Pascal Munoz-Exposito MD, Tobias Tichelbäcker MD, and Aleksandra Kirova MD (211 patients); DZHK (German Center for Cardiovascular Research), partner site Göttingen, Göttingen, Germany: Gerd Hasenfuß MD, Tim Friede MD, Markus Zabel MD, and Simon Schlögl MD; Department of Medical Statistics, University Medical Center Göttingen, Göttingen, Germany: Tim Friede PhD and Markus Harden, PhD; National Heart and Lung Institute, Imperial College, London, United Kingdom: Marek Malik MD, and Katerina Hnatkova PhD; Department of Medical Physiology, University Medical Center Utrecht: Marc A. Vos PhD; Institute for Social Medicine, Epidemiology and Health Economics, Charité Universitätsmedizin Berlin, Berlin, Germany: Stefan N. Willich MD and Thomas Reinhold PhD; University Hospitals of Leuven, Leuven, Belgium: Rik Willems MD and Bert Vandenberk MD (133 patients); Magdalena Klinika, Department of Cardiology, Krapinske Toplice, Croatia: Janko Szavits-Nossan MD and L. Rotkvić MD (124 patients); Attikon University Hospital, 2nd Department of Cardiology, Athens, Greece: Panayota Flevari MD, Andreas Katsimardos MD, and Dimitrios Katsaras MD (112 patients); Slovak Medical University NUSCH, Bratislava, Slovakia: Robert Hatala MD and Martin Svetlosak MD (109 patients); Medical University of Lodz (MUL) WAM Hospital, Department of Cardiology, Lodz, Poland: Andrzej Lubinski MD and Tomasz Kuczejko MD (86 patients); Gentofte Hospital, Copenhagen, Denmark: Jim Hansen MD (81 patients); University Hospital, Department of Cardiology, Basel, Switzerland: Christian Sticherling MD and David Conen MD (74 patients); KBC Sestre Milosrdnice, Department of Cardiology, Zagreb, Croatia: Nikola Pavlović MD, Šime Manola MD, Ozren Vinter MD, and Ivica Benko RN (72 patients); University Medical Center Utrecht, Department of Cardiology/Physiology: Anton Tuinenburg, MD, David Sprenkeler, MD, Agnieszka Smoczynska, MD, and Marc A. Vos PhD (68 patients); University Hospital Tübingen, Department of Cardiology: Axel Bauer MD, Christine Meyer-Zürn MD, and Christian Eick MD (63 patients); Rigshospitalet, The Heart Centre, Department of Cardiology, Copenhagen University Hospital, and Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark: Jesper Hastrup Svendsen MD (61 patients); IDIBAPS, Department of Cardiology, Hospital Clinic Barcelona, Spain: Josep Brugada MD and Elena Arbelo MD (60 patients); SUSSCH, Department of Cardiology, Banska Bystrica, Slovakia: Gabriela Kaliska MD and Jozef Martinek PhD (50 patients); Technische Universität München, Med. Klinik und Poliklinik I, Klinikum rechts der Isar, Munich, Germany: Georg Schmidt MD, Michael Dommasch MD, and Alexander Steger MD (48 patients); Klinikum Großhadern, Department of Cardiology, Ludwig-Maximilians-Universität Munich, Germany: Stefan Kääb MD, Axel Bauer MD, Moritz F. Sinner MD, Konstantinos D Rizas MD, and Wolfgang Hamm MD (44 patients); Acibadem City Clinic Tokuda Hospital, Department of Cardiology: Vassil Traykov MD (42 patients); Medical University of Lodz (MUL), CKD Hospital, Department of Cardiology, Lodz, Poland: Iwona Cygankiewicz MD, Pawel Ptaszyński MD, K. Kaczmarek MD, and I. Poddebska MD (41 patients); St. Ekaterina University Hospital, Department of Cardiology, Sofia, Bulgaria: Svetoslav Iovev MD (41 patients); University Hospital Brno, Department of Internal Medicine and Cardiology, Brno, Czech Republic: Tomáš Novotný MD and Milan Kozak MD (39 patients); Oulu University Hospital and University of Oulu, Medical Research Center, Finland: Heikki Huikuri MD, Tuomas Kenttä MD, and Ari Pelli MSc (36 patients); Bieganski Hospital, Chair and Department of Cardiology, Medical University of Lodz (MUL), Lodz, Poland: Jaroslaw D. Kasprzak MD and Dariusz Qavoq MD (34 patients); KBC Rijeka, Department of cardiovascular disease, Rijeka, Croatia: Sandro Brusich MD, Ervin Avdovic MD, and Marina Klasan RN (33 patients); University Hospital, Department of Cardiology, Olomouc, Czech Republic: Jan Galuszka MD, and Milos Taborsky MD (33 patients); St. Anna Hospital, Department of Cardiology, Sofia, Bulgaria: Vasil Velchev MD (27 patients); Klinikum Reinkenheide Bremerhaven, Department of Cardiology, Germany: Rüdiger Dissmann MD (26 patients); National Heart Hospital, Department of Cardiology, Sofia, Bulgaria:Tchavdar Shalganov MD (22 patients); Poznan Medical University, HSUH Hospital Department of Cardiology, Poznan, Poland: P. Guzik, T. Krauze (19 patients); Marienkrankenhaus Bonn, Department of Cardiology, Germany: Dieter Bimmel MD and Christiane Lieberz RN (19 patients); Klinikum Ludwigsburg, Department of Cardiology, Germany: Stefan Stefanow MD, Norman Rüb MD, and Christian Wolpert MD (19 patients); University Hospital Regensburg, Department of Cardiology, Germany: Joachim Seegers MD and Lars S. Meier MD (18 patients); Vivantes Humboldt Klinikum Berlin, Department of Cardiology: Steffen Behrens MD (17 patients); KBC Split, Department of Cardiology, Croatia: Zrinka Jurisic (12 patients); Karolinska Institutet, Department of Cardiology, Stockholm, Sweden: Frieder Braunschweig MD (12 patients); Charité Campus Virchow Klinikum, Department of Cardiology, Berlin, Germany: Florian Blaschke MD and Burkert Pieske MD (12 patients); General Hospital Zadar, Croatia: Zoran Bakotic MD and Ante Anic MD (10 patients); Klinikum Weiden, Department of Cardiology, Germany: Robert H.G. Schwinger MD (10 patients); Lund University Hospital, Department of Cardiology, Lund, Sweden: Pyotr Platonov MD (2 patients).
Endpoint Adjudication Committee: Gerian Grönefeld MD and Thomas Klingenheben MD.
Funding
This work was supported by the European Community's Seventh Framework Programme FP7/2007-2013 (grant agreement no. HEALTH-F2-2009-602299) for 5 years (starting 1 October 2013).
Conflict of interest: Dr Zabel reports minor travel grants from Biotronik, during the conduct of the study; Dr Willems reports grants from European Community's Seventh Framework Programme FP7/2007-2013, grants from Postdoctoral Clinical Research FWO-Flanders, during the conduct of the study; grants and other from Medtronic, grants and other from Biotronik, other from Abbott, other from Boston Scientific, outside the submitted work; Dr Bauer reports scientific grants and speaker honorarium from Medtronic. Dr Hasenfuss reports personal fees from Berlin Chemie, other from Corvia, personal fees from Impulse Dynamics, personal fees from Novartis, personal fees from Servier, personal fees from Springer, personal fees from Vifor Pharma, outside the submitted work; Dr Svetlosak reports grants from European Union 7th Framework Programme, grant agreement n° 602299, during the conduct of the study; personal fees from Abbott, non-financial support from Boston Scientific , non-financial support from Biotronik, outside the submitted work. Dr Malik reports grants from European Union 7th framework program, during the conduct of the study; Dr Schlögl reports grants from European Union 7th Framework Program, during the conduct of the study. Dr Traykov reports personal fees from Medtronic, personal fees from Pfizer, personal fees from Sandoz, personal fees from Berlin Chemie Menarini, personal fees from Bayer, personal fees from Sopharma, outside the submitted work; Dr Tuinenburg reports grants from European Community's Seventh Framework Programme FP7/2007-2013 (grant agreement n0 HEALTH-F2-2009-602299) for 5 years (starting 1 Oct 2013), during the conduct of the study; Dr Willich reports grants from EU, during the conduct of the study. Harden reports grants from European Commission, during the conduct of the study. Dr Friede reports grants from European Commission, during the conduct of the study; personal fees from Novartis, personal fees from Bayer, personal fees from Janssen, personal fees from SGS, personal fees from Roche, personal fees from Boehringer Ingelheim, personal fees from Daiichi-Sankyo, personal fees from Galapagos, personal fees from Penumbra, personal fees from Parexel, personal fees from Vifor, personal fees from Biosense Webster, personal fees from CSL Behring, personal fees from Fresenius Kabi, personal fees from Coherex Medical, outside the submitted work; Dr Svendsen reports grants, personal fees and other from Medtronic and grants from Gilead, outside the submitted work. Dr Sticherling reports grants from European Union FP7, during the conduct of the study; Dr Merkely reports personal fees from BIOTRONIK, personal fees from Abbott, personal fees from Medtronic, other from Boston Scientific, during the conduct of the study; all other authors have nothing to disclose.
Contributor Information
Markus Zabel, Department of Cardiology and Pneumology, Heart Center, University Medical Center Göttingen, Robert-Koch-Strasse 40, 37075 Göttingen, Germany; DZHK (German Center for Cardiovascular Research), Partner Site Göttingen, Robert-Koch-Str. 42a, 37075 Göttingen, Germany.
Rik Willems, Department of Cardiovascular Sciences, University Hospitals of Leuven, Herestraat 49, 3000 Leuven, Belgium.
Andrzej Lubinski, Department of Cardiology, Medical University of Lodz (MUL) WAM Hospital, ul. Żeromskiego 113, 90-549 Lodz, Poland.
Axel Bauer, Department of Cardiology, Ludwig-Maximilians-Universität Munich, Klinikum Großhadern, Marchioninistr. 19, 81377 München, Germany; DZHK (German Center for Cardiovascular Research), Partner Site Munich Heart Alliance, Biedersteiner Str. 29, 80802 München, Germany; Department of Cardiology, Medical University of Innsbruck, Anichstr. 35, 6020 Innsbruck, Austria.
Josep Brugada, Department of Cardiology, IDIBAPS, Hospital Clinic Barcelona, Carrer de Villaroel, 08036 Barcelona, Spain.
David Conen, Department of Cardiology, University Hospital Basel, University of Basel, Spitalstr. 21, 4031 Basel, Switzerland; Department of Medicine, Population Health Research Institute, McMaster University, 237 Barton Street East, Hamilton, ON LBL 2X2, Canada.
Panagiota Flevari, 2nd Department of Cardiology, Attikon University Hospital, Rimini 1, Chaidari, 12462 Athens, Greece.
Gerd Hasenfuß, Department of Cardiology and Pneumology, Heart Center, University Medical Center Göttingen, Robert-Koch-Strasse 40, 37075 Göttingen, Germany; DZHK (German Center for Cardiovascular Research), Partner Site Göttingen, Robert-Koch-Str. 42a, 37075 Göttingen, Germany.
Martin Svetlosak, Department of Cardiology and Angiology, Slovak Medical University NUSCH, Pod Krasnou horkou 7185, 83101 Nove Mesto, Bratislava, Slovakia.
Heikki V Huikuri, Department of Internal Medicine, Medical Research Center, Oulu University Hospital, University of Oulu, PO Box 8000, 90570 Oulu, Finland.
Marek Malik, National Heart and Lung Institute, Imperial College, Dovehouse Street, London SW36LY, UK.
Nikola Pavlović, Department of Cardiology, KBC Sestre Milosrdnice, Vinogradska Cesta 29, 10000 Zagreb, Croatia.
Georg Schmidt, DZHK (German Center for Cardiovascular Research), Partner Site Munich Heart Alliance, Biedersteiner Str. 29, 80802 München, Germany; Med. Klinik und Poliklinik I, Technische Universität München, Klinikum rechts der Isar, Ismaninger Str. 22, 81675 München, Germany.
Rajevaa Sritharan, Department of Cardiology and Pneumology, Heart Center, University Medical Center Göttingen, Robert-Koch-Strasse 40, 37075 Göttingen, Germany.
Simon Schlögl, Department of Cardiology and Pneumology, Heart Center, University Medical Center Göttingen, Robert-Koch-Strasse 40, 37075 Göttingen, Germany; DZHK (German Center for Cardiovascular Research), Partner Site Göttingen, Robert-Koch-Str. 42a, 37075 Göttingen, Germany.
Janko Szavits-Nossan, Department of Cardiology, Magdalena Klinika, Ul. Ljudevita Gaja 9, 49217 Krapinske Toplice, Croatia.
Vassil Traykov, Department of Cardiology, Acibadem City Clinic Tokuda Hospital, bul. "Nikola Y. Vaptsarov" 51Б, 1407 Sofia, Bulgaria.
Anton E Tuinenburg, Department of Cardiology, University Medical Center Utrecht, Heidelberglaan 100, 3584CX Utrecht, Netherlands.
Stefan N Willich, Institute for Social Medicine, Epidemiology and Health Economics, Charité Universitätsmedizin Berlin, Schumannstr. 20/21, 10117 Berlin, Germany.
Markus Harden, Department of Medical Statistics, University Medical Center Göttingen, Humboldtallee 32, 37073 Göttingen, Germany.
Tim Friede, DZHK (German Center for Cardiovascular Research), Partner Site Göttingen, Robert-Koch-Str. 42a, 37075 Göttingen, Germany; Department of Medical Statistics, University Medical Center Göttingen, Humboldtallee 32, 37073 Göttingen, Germany.
Jesper Hastrup Svendsen, Department of Cardiology, The Heart Centre, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, 2100 København, Copenhagen, Denmark; Department of Clinical Medicine, University of Copenhagen, Blegdamsvej 3B, 2200 København N, Copenhagen, Denmark.
Christian Sticherling, Department of Cardiology, University Hospital Basel, University of Basel, Spitalstr. 21, 4031 Basel, Switzerland.
Béla Merkely, Heart and Vascular Center, Semmelweis University Heart Center, Gaál József út 9, 1122 Budapest, Hungary.
EU-CERT-ICD Study Investigators:
Béla Merkely, Peter Perge, Zoltan Sallo, Gabor Szeplaki, Nandor Szegedi, Klaudia Vivien Nagy, Markus Zabel, Lars Lüthje, Simon Schlögl, R Sritharan, Helge Haarmann, Leonard Bergau, Joachim Seegers, Gerd Hasenfuß, Pascal Munoz-Exposito, Tobias Tichelbäcker, Aleksandra Kirova, Gerd Hasenfuß, Tim Friede, Markus Zabel, Simon Schlögl, Tim Friede, Markus Harden, Marek Malik, Katerina Hnatkova, Marc A Vos, Stefan N Willich, Thomas Reinhold, Rik Willems, Bert Vandenberk, Magdalena Klinika, Janko Szavits-Nossan, L Rotkvić, Panayota Flevari, Andreas Katsimardos, Dimitrios Katsaras, Robert Hatala, Martin Svetlosak, Andrzej Lubinski, Tomasz Kuczejko, Jim Hansen, Christian Sticherling, David Conen, Nikola Pavlović, Šime Manola, Ozren Vinter, Ivica Benko, Anton Tuinenburg, David Sprenkeler, A Smoczynska, M A Vos, Axel Bauer, Christine Meyer-Zürn, Christian Eick, Jesper Hastrup Svendsen, Josep Brugada, Elena Arbelo, Gabriela Kaliska, Jozef Martinek, Georg Schmidt, Michael Dommasch, Alexander Steger, Stefan Kääb, Axel Bauer, Moritz F Sinner, Konstantinos D Rizas, Wolfgang Hamm, V Traykov, Iwona Cygankiewicz, Pawel Ptaszyński, K Kaczmarek, I Poddebska, Svetoslav Iovev, Tomáš Novotný, Milan Kozak, Heikki Huikuri, Tuomas Kenttä, Ari Pelli, Jaroslaw D Kasprzak, Dariusz Qavoq, Sandro Brusich, Ervin Avdovic, Marina Klasan, Jan Galuszka, Milos Taborsky, Vasil Velchev, Rüdiger Dissmann, T Shalganov, P Guzik, T Krauze, Dieter Bimmel, Christiane Lieberz, Klinikum Ludwigsburg, Stefan Stefanow, Norman Rüb, Christian Wolpert, Joachim Seegers, Lars S Meier, Steffen Behrens, Zrinka Jurisic, Frieder Braunschweig, Florian Blaschke, Burkert Pieske, Zoran Bakotic, Ante Anic, Klinikum Weiden, Robert H G Schwinger, Pyotr Platonov, Gerian Grönefeld, and Thomas Klingenheben
References
- 1. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 2. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 3. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigator. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151–2158. [DOI] [PubMed] [Google Scholar]
- 4. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 5. Raatikainen MJ, Arnar DO, Merkely B, Camm AJ, Hindricks G. Access to and clinical use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2016 Report from the European Heart Rhythm Association. Europace 2016;18:iii1–iii79. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt M, Ulrichsen SP, Pedersen L, Botker HE, Sorensen HT. Thirty-year trends in heart failure hospitalization and mortality rates and the prognostic impact of co-morbidity: a Danish nationwide cohort study. Eur J Heart Fail 2016;18:490–499. [DOI] [PubMed] [Google Scholar]
- 7. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp-Pedersen C, Pehrson S; DANISH Investigators. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 8. Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for Medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol 2012;60:1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koller MT, Schaer B, Wolbers M, Sticherling C, Bucher HC, Osswald S. Death without prior appropriate implantable cardioverter-defibrillator therapy: a competing risk study. Circulation 2008;117:1918–1926. [DOI] [PubMed] [Google Scholar]
- 10. Goldberger JJ, Buxton AE. Personalized medicine vs guideline-based medicine. JAMA 2013;309:2559–2560. [DOI] [PubMed] [Google Scholar]
- 11. Lee DS, Hardy J, Yee R, Healey JS, Birnie D, Simpson CS, Crystal E, Mangat I, Nanthakumar K, Wang X, Krahn AD, Dorian P, Austin PC, Tu JV; Investigators of the Ontario ICD Database. Clinical risk stratification for primary prevention implantable cardioverter defibrillators. Circ Heart Fail 2015;8:927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergau L, Willems R, Sprenkeler DJ, Fischer TH, Flevari P, Hasenfuß G, Katsaras D, Kirova A, Lehnart SE, Lüthje L, Röver C, Seegers J, Sossalla S, Dunnink A, Sritharan R, Tuinenburg AE, Vandenberk B, Vos MA, Wijers SC, Friede T, Zabel M. Differential multivariable risk prediction of appropriate shock versus competing mortality—a prospective cohort study to estimate benefits from ICD therapy. Int J Cardiol 2018;272:102–107. [DOI] [PubMed] [Google Scholar]
- 13. Zabel M, Sticherling C, Willems R, Lubinski A, Bauer A, Bergau L, Braunschweig F, Brugada J, Brusich S, Conen D, Cygankiewicz I, Flevari P, Taborsky M, Hansen J, Hasenfuß G, Hatala R, Huikuri HV, Iovev S, Kääb S, Kaliska G, Kasprzak JD, Lüthje L, Malik M, Novotny T, Pavlović N, Schmidt G, Shalganov T, Sritharan R, Schlögl S, Szavits Nossan J, Traykov V, Tuinenburg AE, Velchev V, Vos MA, Willich SN, Friede T, Svendsen JH, Merkely B; EU-CERT-ICD Investigators. Rationale and design of the EU-CERT-ICD prospective study: comparative effectiveness of prophylactic ICD implantation. ESC Heart Fail 2019;6:182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heinze G, Juni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J 2011;32:1704–1708. [DOI] [PubMed] [Google Scholar]
- 15. Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, Nichols M, Stone GW, Pocock SJ. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol 2017;69:345–357. [DOI] [PubMed] [Google Scholar]
- 16. Seegers J, Conen D, Jung K, Bergau L, Dorenkamp M, Luthje L, Sohns C, Sossalla ST, Fischer TH, Hasenfuss G, Friede T, Zabel M. Sex difference in appropriate shocks but not mortality during long-term follow-up in patients with implantable cardioverter-defibrillators. Europace 2016;18:1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics 1983;39:499–503. [PubMed] [Google Scholar]
- 18. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA 3rd, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W; MADIT-RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–2283. [DOI] [PubMed] [Google Scholar]
- 19. Sticherling C, Arendacka B, Svendsen JH, Wijers S, Friede T, Stockinger J, Dommasch M, Merkely B, Willems R, Lubinski A, Scharfe M, Braunschweig F, Svetlosak M, Zurn CS, Huikuri H, Flevari P, Lund-Andersen C, Schaer BA, Tuinenburg AE, Bergau L, Schmidt G, Szeplaki G, Vandenberk B, Kowalczyk E, Eick C, Juntilla J, Conen D, Zabel M; EU-CERT-ICD Investigators. Sex differences in outcomes of primary prevention implantable cardioverter-defibrillator therapy: combined registry data from eleven European countries. Europace 2018;20:963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med 2002;21:2917–2930. [DOI] [PubMed] [Google Scholar]
- 21. Sharma A, Al-Khatib SM, Ezekowitz JA, Cooper LB, Fordyce CB, Michael Felker G, Bardy GH, Poole JE, Thomas Bigger J, Buxton AE, Moss AJ, Friedman DJ, Lee KL, Steinman R, Dorian P, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Peterson ED, Inoue LYT, Sanders GD. Implantable cardioverter-defibrillators in heart failure patients with reduced ejection fraction and diabetes. Eur J Heart Fail 2018;20:1031–1038. [DOI] [PubMed] [Google Scholar]
- 22. Junttila MJ, Pelli A, Kentta TV, Friede T, Willems R, Bergau L, Malik M, Vandenberk B, Vos MA, Schmidt G, Merkely B, Lubinski A, Svetlosak M, Braunschweig F, Harden M, Zabel M, Huikuri HV, Sticherling C; EU-CERT-ICD Investigators. Appropriate shocks and mortality in patients with versus without diabetes with prophylactic implantable cardioverter defibrillators. Diabetes Care 2020;43:196–200. [DOI] [PubMed] [Google Scholar]
- 23. Elming MB, Nielsen JC, Haarbo J, Videbæk L, Korup E, Signorovitch J, Olesen LL, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp-Pedersen C, Pehrson S, Køber L, Thune JJ. Age and outcomes of primary prevention implantable cardioverter-defibrillators in patients with nonischemic systolic heart failure. Circulation 2017;136:1772–1780. [DOI] [PubMed] [Google Scholar]
- 24. Bergau L, Seegers J, Zabel M. Sex differences in ICD benefit. J Electrocardiol 2014;47:869–873. [DOI] [PubMed] [Google Scholar]
- 25. Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, Maggioni AP, Anand I, Poole-Wilson PA, Fishbein DP, Johnson G, Anderson J, Mark DB, Bardy GH. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation 2009;120:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012;59:2075–2079. [DOI] [PubMed] [Google Scholar]
- 27. Friedman DJ, Al-Khatib SM, Zeitler EP, Han J, Bardy GH, Poole JE, Bigger JT, Buxton AE, Moss AJ, Lee KL, Steinman R, Dorian P, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Inoue LYT, Sanders GD. New York Heart Association class and the survival benefit from primary prevention implantable cardioverter defibrillators: a pooled analysis of 4 randomized controlled trials. Am Heart J 2017;191:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bilchick KC, Wang Y, Cheng A, Curtis JP, Dharmarajan K, Stukenborg GJ, Shadman R, Anand I, Lund LH, Dahlström U, Sartipy U, Maggioni A, Swedberg K, O’Conner C, Levy WC. Seattle heart failure and proportional risk models predict benefit from implantable cardioverter-defibrillators. J Am Coll Cardiol 2017;69:2606–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santangeli P, Di Biase L, Dello Russo A, Casella M, Bartoletti S, Santarelli P, Pelargonio G, Natale A. Meta-analysis: age and effectiveness of prophylactic implantable cardioverter-defibrillators. Ann Intern Med 2010;153:592–599. [DOI] [PubMed] [Google Scholar]
- 30. Zeitler EP, Hellkamp AS, Fonarow GC, Hammill SC, Curtis LH, Hernandez AF, Al-Khalidi HR, Curtis JP, Heidenreich PA, Anstrom KJ, Peterson ED, Mark DB, Hammill BG, Sanders GD, Al-Khatib SM. Primary prevention implantable cardioverter-defibrillators and survival in older women. JACC Heart Fail 2015;3:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle heart failure model: prediction of survival in heart failure. Circulation 2006;113:1424–1433. [DOI] [PubMed] [Google Scholar]
- 32. Kramer DB, Friedman PA, Kallinen LM, Morrison TB, Crusan DJ, Hodge DO, Reynolds MR, Hauser RG. Development and validation of a risk score to predict early mortality in recipients of implantable cardioverter-defibrillators. Heart Rhythm 2012;9:42–46. [DOI] [PubMed] [Google Scholar]
- 33. Rizas KD, McNitt S, Hamm W, Massberg S, Kaab S, Zareba W, Couderc JP, Bauer A. Prediction of sudden and non-sudden cardiac death in post-infarction patients with reduced left ventricular ejection fraction by periodic repolarization dynamics: MADIT-II substudy. Eur Heart J 2017;38:2110–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bauer A, Klemm M, Rizas KD, Hamm W, von Stülpnagel L, Dommasch M, Steger A, Lubinski A, Flevari P, Harden M, Friede T, Kääb S, Merkely B, Sticherling C, Willems R, Huikuri H, Malik M, Schmidt G, Zabel M; EU-CERT-ICD Investigators. Periodic repolarization dynamics predict the mortality benefit in patients undergoing prophylactic implantation of a defibrillator: a prospective controlled multicentre cohort study. Lancet 2019;394:1344–1351. [DOI] [PubMed] [Google Scholar]
- 35. Dagres N, Hindricks G. Devices for management of sudden cardiac death: successes, challenges and perspectives. Int J Cardiol 2017;237:34–37. [DOI] [PubMed] [Google Scholar]
- 36. van Barreveld M, Dijkgraaf MGW, Hulleman M, Boersma LVA, Delnoy P, Meine M, Tuinenburg AE, Theuns D, van der Voort PH, Kimman GP, Buskens E, Tijssen JPG, Bruinsma N, Verstraelen TE, Zwinderman AH, van Dessel P, Wilde AAM; DO-IT Investigators. Dutch outcome in implantable cardioverter-defibrillator therapy (DO-IT): registry design and baseline characteristics of a prospective observational cohort study to predict appropriate indication for implantable cardioverter-defibrillator. Neth Heart J 2017;25:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.