Abstract
Plants monitor seasonal cues to optimize reproductive success by tuning onset of reproduction and inflorescence architecture. TERMINAL FLOWER 1 (TFL1) and FLOWERING LOCUS T (FT) and their orthologs antagonistically regulate these life history traits, yet their mechanism of action, antagonism and targets remain poorly understood. Here, we show that TFL1 is recruited to thousands of loci by the bZIP transcription factor FD. We identify the master regulator of floral fate, LEAFY (LFY) as a target under dual opposite regulation by TFL1 and FT and uncover a pivotal role of FT in promoting flower fate via LFY upregulation. We provide evidence that the antagonism between FT and TFL1 relies on competition for chromatin-bound FD at shared target loci. Direct TFL1-FD regulated target genes identify this complex as a hub for repressing both master regulators of reproductive development and endogenous signalling pathways. Our data provide mechanistic insight into how TFL1-FD sculpt inflorescence architecture, a trait important for reproductive success, plant architecture and yield.
Subject terms: Developmental biology, Plant sciences
The flowering regulators TFL1 and FT act antagonistically to synchronize reproductive transition in flowering plants. Here Zhu et al. show that TFL1 is recruited by the bZIP transcription factor FD to thousands of target loci and reveal that FT competes with TFL1 for chromatin bound-FD at shared targets including LFY, upregulation of which by FT promotes floral fate.
Introduction
Of particular importance for reproductive success of flowering plants is optimal timing of onset of reproductive development and of the transition from branch to floral fate in the inflorescence in response to seasonal cues1–5. For example, in plants that flower only once, like Arabidopsis and most crops, an early switch to flower formation allows rapid completion of the life-cycle and is beneficial in a short growing season5–7. At the same time early onset of flower formation reduces seed set and yield since flowers form in lieu of branches, which support production of more flowers per plant5–8. By contrast, delaying flower formation increases branching and total flower number, but prolongs time to seed set5–8.
Key regulators of seasonal control of onset of reproductive development and of the switch from branch to floral fate in primordia of the inflorescence are members of the phosphatidylethanolamine-binding protein (PEBP) family of proteins5,6,9,10. Among these, FT promotes onset of the reproductive phase and flower formation (determinacy), while TFL1 promotes vegetative development and branch fate (indeterminacy)9,11–13. Arabidopsis flowers in the spring and FT accumulates when the daylength exceeds a critical threshold, while TFL1 is present in both short-day and long-day conditions2,3,14.
FT and TFL1 are small mobile proteins, which have been implicated in transcriptional regulation but do not have DNA-binding domains14–18. Biochemical and genetic studies showed that FT physically interacts with the bZIP transcription factor FD via 14-3-3 proteins and similar interactions have recently been described for TFL119–22. Indeed, despite their antagonistic roles, TFL1 and FT are distinguished by only a small number of nonconservative amino acid changes11,12,23,24. FT can be converted into TFL1 and vice versa by a single amino acid substitution and such mutations have been selected for during crop domestication23–26. Accumulating evidence suggests that FT acts as a transcriptional co-activator, while TFL1 may either prevent FT activity or act as a co-repressor23,27. However, non-nuclear roles have also been described for both TFL1 and FT28,29.
A key unanswered question is how the florigens modulate plant form—what are the downstream processes they set in motion and what is molecular basis for their antagonism? Here we show that TFL1 is recruited to target loci by the bZIP transcription factor FD. We identify the master regulator of floral fate, LEAFY, as a target under dual opposite regulation by TFL1 and FT and uncover a prominent role for FT in LFY upregulation. We find that the antagonism between TFL1 and FT relies on competition for access to chromatin bound FD at the LFY locus and other shared targets. Finally, we identify hundreds of TFL1–FD regulated genes linking this complex not only to repression of master regulators of floral fate, but also of diverse endogenous signalling pathways. The combined data reveals how TFL1 and FT tune inflorescence architecture in response to seasonal cues by altering transcriptional programs that direct primordium fate in the inflorescence.
Result
TFL1 is recruited to thousands of loci by the bZIP transcription factor FD
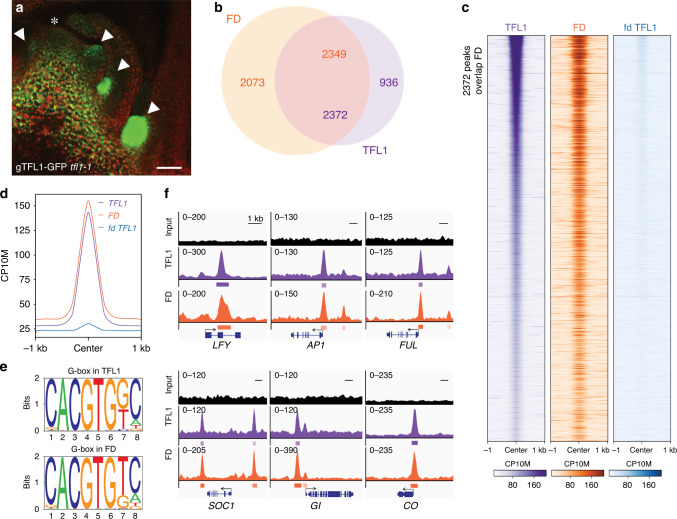
Mechanistic insight into TFL1 activity has been hampered by low protein abundance. To overcome this limitation and to test the role of TFL1 in the nucleus, we first generated a biologically active, genomic GFP-tagged version of TFL1 (gTFL1-GFP tfl1-1) (Supplementary Fig. 1a–c) and identified a developmental stage and tissue where TFL1 accumulates. TFL1 protein strongly accumulated in branch meristems in the axils of cauline leaves in 42-day-old short-day grown plants just prior to the switch to flower formation (Fig. 1a). To conduct TFL1 chromatin immunoprecipitation followed by sequencing (ChIP-seq), we next isolated shoot apices at this stage for anti-GFP immunoprecipitation. Because TFL1 is present in very few cells and binds chromatin indirectly, we combined eight individual ChIP reactions per replicate to enhance detection. We conducted FD ChIP-seq in analogous fashion using a published, biologically active, genomic fusion protein (gFD-GUS fd-1)20 (Supplementary Fig. 1d). This approach yielded high-quality ChIP-seq data in both cases (Supplementary Figs. 2 and 3a).
Fig. 1. TFL1 is recruited by FD to target loci.
a gTFL1-GFP expression in branch meristems (arrowheads) in the axils of cauline leaves in 42-day-old short-day grown plants. Asterisk: shoot apex. b Overlap of significant (MACS2 q value ≤ 10−10) FD and TFL1 ChIP-seq peaks. Numbers in the intersect show FD peaks overlapping with TFL1 peaks (orange) and TFL1 peaks overlapping with FD peaks (purple). c, d Comparison of ChIP-seq datasets. Heatmaps of TFL1, FD and TFL1(fd) ChIP-seq peaks (c). All heatmaps are centred on TFL1-binding peak summits and ranked from the lowest to the highest TFL1 summit signal. CP10M: counts per 10 million reads. ChIP-seq signal at TFL1 summits for TFL1 in the wild-type or in fd mutants and for FD (d). e Most significant motifs identified by de novo motif analysis under TFL1 or FD peak summits. f Browser view of TFL1 and FD-binding peaks at genes that promote onset of reproductive development3 (SOC1, GI, CO) or the switch to flower fate32 (LFY, AP1, FUL). Significant peaks (summit q value ≤ 10−10) according to MACS2 are marked by horizontal bars, with the colour saturation proportional to the negative log 10 q value (as for the narrowPeak file format in ENCODE). See also Supplementary Figs. 1–3 and Supplementary Data 1.
In total, we identified 3308 and 4422 significant TFL1 and FD peaks (MACS2 summit qval ≤ 10−10), respectively (Fig. 1b). The TFL1 peaks significantly overlapped with the FD peaks (72% overlap, p val < 10−300, hypergeometric test; Fig. 1b–d). De novo motif analysis of ChIP peak summits identified the G-box cis motif, a known FD-binding site30, as most significantly enriched (p val < 10−470) and frequently present (>84%) under TFL1 bound and TFL1/FD co-bound peaks (Fig. 1e and Supplementary Fig. 2). To test whether TFL1 chromatin occupancy is dependent on the presence of FD, we also performed TFL1 ChIP-seq in the fd-1 null mutant. TFL1 chromatin occupancy was strongly reduced in fd-1 (Fig. 1c, d). Our data point to a prominent nuclear role for TFL1 and show that FD recruits TFL1 to the chromatin of target loci.
Annotating FD and TFL1 peaks to genes identified 2699 joint TFL1 and FD targets. Gene Ontology (GO) term enrichment analysis implicates these targets in abiotic and endogenous stimulus response and reproductive development (Supplementary Table 1). TFL1 and FD peaks were present at loci that promote onset of the reproductive phase in response to inductive photoperiod2,3,31 like GIGANTEA (GI), CONSTANS (CO), and SUPPRESSOR OF CONSTANS 1 (SOC1) and at loci that promote floral fate31,32 such as LFY, APETALA1 (AP1), and FRUITFULL (FUL) (Fig. 1f). Identification of these TFL1 and FD co-bound targets fits with the known biological role of TFL1 as a suppressor of onset of reproduction and of flower fate and the proposed molecular function of TFL1 in opposing gene activation11–13,27.
LEAFY is under dual opposite regulation by TFL1/FD and FT/FD
We selected the LEAFY (LFY) gene, which encodes a master regulator of flower fate33,34, to further probe the molecular mechanism of action of TFL1. While TFL1 promotes branch fate, LFY promotes flower fate in primordia (Supplementary Fig. 4a–f)13,33–35. Using independent biological replicates, we confirmed FD-mediated TFL1 binding to LFY by ChIP-qPCR (Supplementary Fig. 4g, h). To test whether LFY expression is rapidly repressed by the TFL1–FD complex, we generated transgenic plants expressing a steroid inducible version of TFL1 (TFL1ER; Supplementary Fig. 5). A single steroid treatment reduced LFY levels by 50% after 4 h (Supplementary Fig. 4i). The combined data suggest that the TFL1–FD complex directly represses LFY.
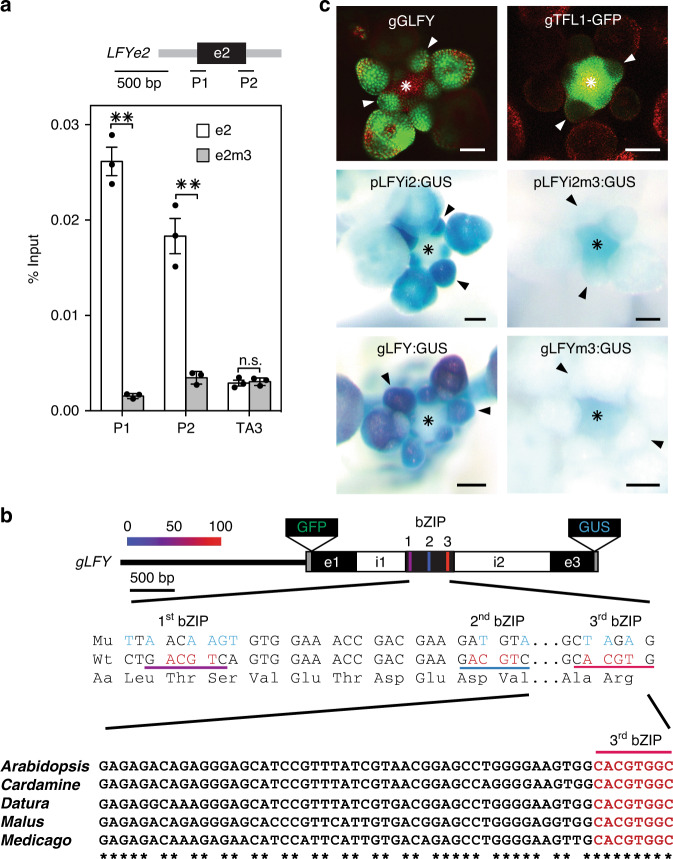
To better understand TFL1 recruitment to the LFY locus, we identified the genomic region sufficient and the cis motifs necessary for TFL1 association with the LFY locus. TFL1 and FD peak summits located to the second exon of LFY (Fig. 1f and Supplementary Fig. 4g, h) and LFY reporters that lacked the second exon were not repressed in response to TFL1 overexpression (Supplementary Fig. 6a, b). Exonic transcription factor-binding sites, although rare, are found in both animals and plants, and frequently link to developmental regulation36,37. To test whether LFY exon 2 (e2) alone is sufficient to recruit TFL1–FD, we transformed gTFL1-GFP tfl1-1 plants with a T-DNA containing only LFY e2. We detected strong TFL1 recruitment to the introduced copy of e2, using primer sets that specifically amplify the transgene borne exon (Fig. 2a). Next we identified three putative bZIP-binding sites in the second exon of LFY; These include an evolutionarily conserved G-box and two partially conserved C-boxes (Fig. 2b). When we transformed gTFL1-GFP tfl1-1 with a version of LFY e2 in which the three bZIP binding were mutated (e2m3), we were unable to detect TFL1 binding to the introduced copy of e2m3 (Fig. 2a). The three bZIP-binding sites in the second exon of LFY are thus necessary for TFL1 recruitment. Similar results were obtained when we tested TFL1 recruitment to e2 and e2m3 via FD in yeast (Supplementary Fig. 6c).
Fig. 2. Exonic bZIP cis motifs mediate TFL1 recruitment to LFY.
a TFL1 recruitment to LFY exon 2 (e2) or a bZIP-binding site mutated version thereof (e2m3) in 42-day-old short-day-grown plants. Top: LFY exon 2 (e2, black rectangle) and T-DNA vector (grey line). Centre: location of amplicons P1 and P2, each consisting of one exon 2-specific and one vector-specific primer. Bottom: gTFL1-GFP ChIP-qPCR. Progeny pools of >50 random gTFL1-GFP T1 plants transformed with e2 or e2m3 were analyzed. Shown are mean ± SEM of three independent biological experiments (black dots). P values unpaired one-tailed t-test: **P1 = 0.0018, **P2 = 0.0078; n.s. TA3 = 0.35. b Top: genomic LFY construct with GFP (gGLFY) or beta-glucuronidase (gLFY-GUS). Putative bZIP-binding motifs in LFY exon 2 are colour-coded based on conservation. For the pLFYi2:GUS reporter diagram see Supplementary Fig. 6a. Centre: mutation of the three bZIP-binding motifs without changing the primary amino acid sequence. Bottom: evolutionary conservation of the G-box (3rd bZIP). c Top: expression domain of LFY and TFL1 proteins in inflorescence apices with flower primordia. Centre and Bottom: accumulation of beta-glucuronidase in a wild-type or bZIP-binding site mutated (m3) reporter (pLFYi2:GUS) or genomic construct (gLFY-GUS). Plants were grown in long day. Staining was conducted under identical conditions. Arrowheads: flower primordia; asterisk: inflorescence shoot apex; Scale bars: 2 mm. See also Supplementary Figs. 4–6.
Having identified the cis motifs necessary for TFL1 recruitment to LFY, we next probed their contribution to spatiotemporal LFY accumulation. LFY reporters that contain e2 (pLFYi2-GUS, Supplementary Fig. 6a) and a genomic LFY reporter (gLFY-GUS, Fig. 2b) recapitulated endogenous LFY expression (Fig. 2c, Supplementary Fig. 6d)34,35. Mutating the three bZIP-binding sites in pLFYi2-GUS or gLFY-GUS caused ectopic reporter expression in the centre of the inflorescence shoot apex (Fig. 2c, Supplementary Fig. 6d). This is the precise region where TFL1 protein accumulates during reproductive development (Fig. 2c)14. Indeed, LFY is known to be ectopically expressed in the inflorescence shoot apex of tfl1 mutants during reproductive development13. Thus, TFL1–FD binding to the bZIP motifs of e2 of LFY is required to prevent ectopic LFY accumulation in the centre of the shoot apex.
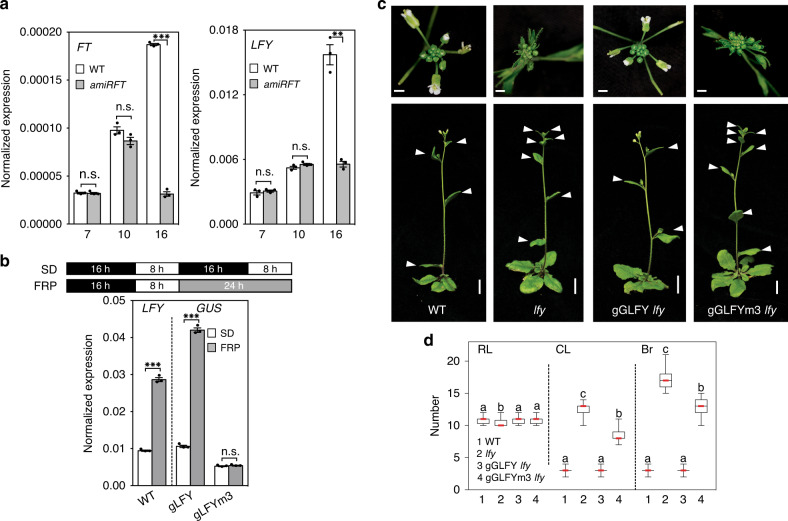
Surprisingly, the bZIP-binding site mutations in the second exon of LFY in addition strongly reduced reporter expression in incipient and young flower primordia (Fig. 2c, Supplementary Fig. 6d). This suggests that the bZIP motifs may be required for LFY upregulation in these flower primordia, perhaps via FT. Based on prior studies31,38–41, LFY was not thought to be an immediate early FT target. Because constitutive mutants that delay onset of the reproductive phase, like ft, indirectly delay the switch to flower formation42 we wished to deplete FT specifically during the reproductive phase to test whether FT promotes LFY expression. Towards this end, we used a minimal FT promoter (p4kbFT) that is active in parts of leaves and stems in long-day grown plants only after day 1243. We fused p4kbFT to a previously characterized FT-specific artificial microRNA44. In the resulting conditional ft mutant (p4kbFT:amiRFT), onset of reproduction was not delayed (Fig. 3a, Supplementary Fig. 7). However, p4kbFT:amiRFT plants displayed a significantly delay in onset of flower formation and failed to upregulate LFY expression (Fig. 3a and Supplementary Fig. 7).
Fig. 3. FT upregulates LFY expression to promote floral fate.
a Expression of FT and LFY in above-ground tissues of long-day-grown wild-type (WT) and pFT4kb:amiRFT plants prior to onset of (day 7 and day 10) or during (day 16) reproductive development. b Effect of a single far-red enriched photoperiod (FRP) on LFY (left) and reporter (GUS, right) accumulation in 42-day-old short day (SD) grown plants. a, b Expression was normalized over UBQ10. Shown are mean ± SEM of three independent biological experiments (black dots). Unpaired one-tailed t-test; p values: n.s. FT day 7 = 0.407, n.s. FT day 10 = 0.052, ***FT day 16 = 6E−05; n.s. LFY day 7 = 0.258, n.s. LFY day 10 = 0.07, **LFY day 16 = 0.0045 (a) ***LFY WT ± FRP = 0.0004, ***gLFY-GUS ± FRP = 0.0001, n.s. gLFY-GUSm3 ± FRP = 0.217 (b). c Rescue of lfy-1 null mutants by genomic GFP-tagged LFY (gGLFY) or a bZIP-binding site mutated version thereof (gGLFYm3) in long-day-grown plants. Representative inflorescence images (top and side view). Arrowheads indicate branches formed on the main stem. For m3 mutations see Fig. 2a. d Phenotype quantification of 15 independent transgenic lines for gGLFY lfy and gGLFYm3 lfy. RL rosette leaves, CL cauline leaves, Br branches. Box plot-median (red line), upper and lower quartiles (box edges), and minima and maxima (whiskers). Letters: significantly different groups p value < 0.05 based on Kruskal–Wallis test with Dunn’s post hoc test. Scale bars, 1 cm. See also Supplementary Figs. 6–10.
In a parallel approach to test the role of FT in LFY induction, we induced endogenous FT expression by treating 42-day-old short-day grown plants with a single far-red-enriched long-day photoperiod (FRP). Far-red light enhances FT induction by photoperiod (Supplementary Fig. 8a, b)45,46. In addition, FRP triggered significant LFY induction, which was dependent on the presence of FT (Supplementary Fig. 8c, d). A single FRP treatment also induced the gLFY-GUS reporter, but only if the bZIP-binding sites in e2 were intact (Fig. 3b). Finally, we probed for rapid LFY induction by FT after generating an estradiol inducible version of FT (35S:FT-HAER) (Supplementary Fig. 9a–c). A single steroid treatment triggered significant LFY induction after 4 h (Supplementary Fig. 9d). After crossing FT-HAER to LFY reporters containing (pLFYi2:GUS) or lacking (pLFYi2m3:GUS) the bZIP-binding sites in e2, we tested reporter activity in response to steroid activation. GUS upregulation was similar to that of endogenous LFY when the bZIP-binding sites were present (Supplementary Fig. 9e). By contrast, GUS expression was not upregulated in the pLFYi2m3:GUS FT-HAER plants after steroid induction (Supplementary Fig. 9e). The combined loss-of-function, photoinduction and gain-of-function data indicate that FT–FD directly activates LFY expression via bZIP-binding sites in the second exon.
These findings prompted us to assess the biological importance of the LFY bZIP-binding sites for inflorescence architecture. While a genomic GFP-tagged LFY construct (gGLFY) fully rescued the lfy-1 null mutant (in 24 out of 25 independent transgenic lines), a construct which preserves LFY protein sequence but has mutated bZIP-binding sites (gGLFYm3) yielded only partial rescue (in 15 out of 15 independent transgenic lines) (Fig. 3c, d and Supplementary Fig. 10). In the gGLFYm3 lfy-1 plants onset of flower formation was significantly delayed, leading to formation of many more branches, and LFY accumulation was strongly reduced (Fig. 3c, d and Supplementary Fig. 10). The dramatic reduction of LFY accumulation is striking given the many additional positive inputs into LFY upregulation previously identified38,40,47,48. Our combined data uncover a pivotal role of FT in LFY upregulation and reveal that FT promotes flower formation via LFY. We note that LFY accumulation in the centre of the gGLFYm3 lfy-1 shoot apex was much lower than that observed in tfl1 mutants and that gGLFYm3 lfy-1 did not exhibit the terminal flower phenotype typical of tfl1 (Supplementary Fig. 10)13. This is expected since the bZIP mutations at the LFY locus prevent access of both TFL1 and of activating PEBP family members FT and the closely related TWIN SISTER OF FT (TSF). Indeed, it has been shown that the terminal flower phenotype of tfl1 is suppressed in tfl1 ft tsf triple mutants49.
FT competes TFL1 from FD bound at shared target loci
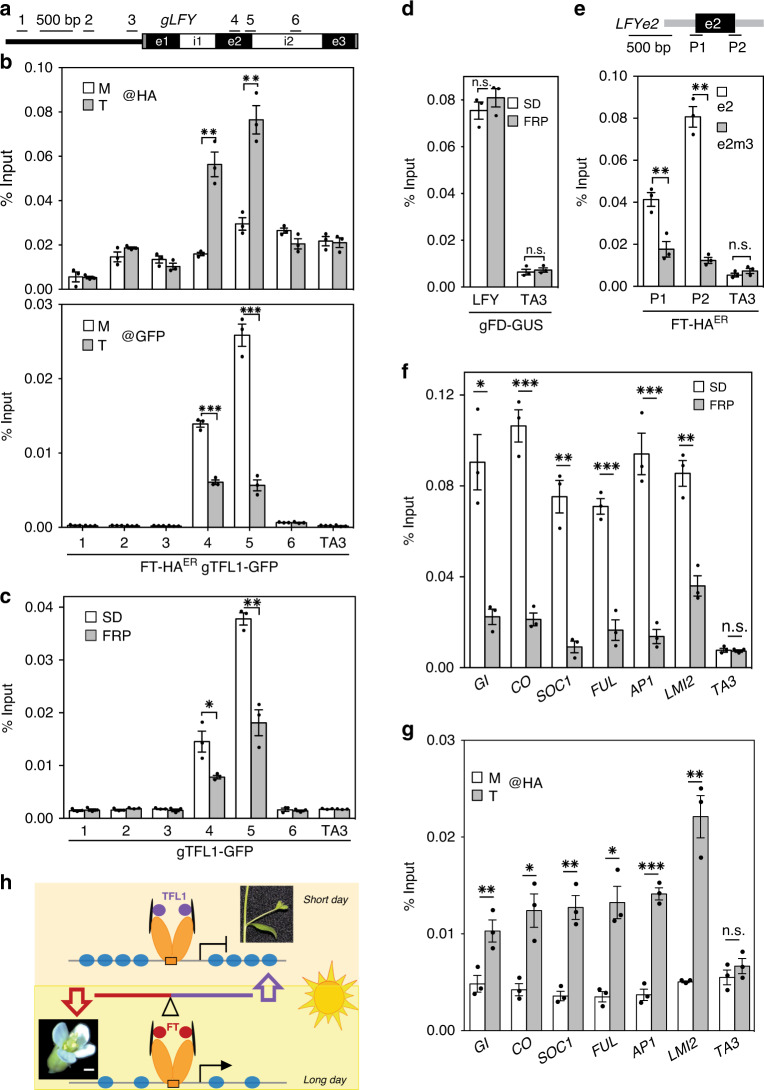
Having identified LFY as a target under dual opposite regulation by TFL1 and FT, we next investigated the mechanism underlying the TFL1–FT antagonism at this locus. To test for possible competition between FT and TFL1 at the chromatin, we conducted anti-HA ChIP-qPCR in 42-day-old short-day grown FT-HAER gTFL1-GFP plants four hours after mock or steroid application. Estradiol induction led to rapid recruitment of FT-HA to the second exon of LFY, the region occupied by FD and TFL1 (compare Fig. 4a, b to Supplementary Fig. 4h). Anti-GFP ChIP-qPCR performed on the same sample uncovered a concomitant reduction in TFL1 occupancy (Fig. 4a, b). We next asked whether upregulation of endogenous FT also triggers reduced TFL1 occupancy at the LFY locus. Towards this end, we treated plants with a single FRP to upregulate FT (Supplementary Fig. 8b). The single FRP likewise significantly reduced TFL1 occupancy at the LFY chromatin (Fig. 4c). By contrast, photoinduction of FT did not alter FD occupancy (Fig. 4d). To probe whether FT is recruited to the second exon of LFY via the bZIP-binding sites, we transformed FT-HAER with either a wild-type version of e2 or a bZIP-binding site mutated version thereof (e2m3). After steroid induction, we used transgene-specific primers to monitor FT binding to the two versions of LFY e2 by ChIP-qPCR. As described above for TFL1 (Fig. 2a), FT was recruited to LFY e2 alone (Fig. 4e). In addition, FT recruitment to the introduced copy of LFY e2 was abolished when the three bZIP-binding sites were mutated (Fig. 4e).
Fig. 4. FT competes TFL1 from the chromatin.
a LFY locus and primers used. b Top: FT-HAER occupancy at the LFY locus after 4-h mock (M) or steroid (T) treatment. Bottom: gTFL1 occupancy in the same sample. c, d Effect of FT upregulation by photoperiod (FRP, 24 h) on TFL1 (c) or FD (d) occupancy at the LFY locus. e FT-HAER recruitment to LFY exon 2 (e2) or a bZIP-binding site mutated version thereof (e2m3) after 4-hour steroid treatment. Top: LFY exon 2 (e2, black rectangle) and T-DNA vector (grey line). Centre: location of amplicons P1 and P2, each consisting of one exon 2-specific and one vector-specific primer. Below: FT-HAER ChIP-qPCR. Progeny pools of >50 random FT-HAER T1 plants transformed with e2 or e2m3 were analyzed (see also ref. 108). f Effect of photoperiod (FRP) on TFL1 occupancy at TFL1–FD target loci identified in Fig. 1f and LMI267 g FT-HAER occupancy with or without 4-h estradiol treatment at TFL1–FD bound regions of target loci shown in f. b–g ChIP was performed in 42-day-old short-day-grown plants. Shown are mean ± SEM of three independent biological experiments (black dots). P values (unpaired one-tailed t-test): b anti-HA ChIP ** region 4 = 0.009, region 5 = 0.003; anti-GFP ChIP ***region 4 = 6E−05, region 5 = 0.006; c *region 4 = 0.039, **; region 5 = 0.002; d n.s. LFY = 0.18, TA3 = 0.29. e **P1 = 0.004, P2 = 0.002, n.s. TA3 = 0.12; f * GI = 0.016, ***CO = 0.0007, **SOC1 = 0.0015, ***FUL = 0.0003, ***AP1 = 0.007, LMI2 = 0.0011, n.s. TA3 = 0.34. g **GI = 0.009, *CO = 0.011, **SOC1 = 0.003, *FUL = 0.015, ***AP1 = 0.0001, **LMI2 = 0.008, n.s TA3 = 0.17. h Model for antagonistic roles of TFL1 (purple circles) and FT (red circles) in promoting branch fate or floral fate, respectively. Increased FT accumulation leads to competition of TFL1 from bZIP transcription factor FD bound to chromatin and to onset of flower formation. FD dimers (orange ovals), 14-3-3 proteins (black disks).
Our combined data suggest that FT competes TFL1 from FD bound at exonic bZIP motifs at the LFY locus. The loss of TFL1 from the LFY locus via competition by FT is further supported by the finding that neither steroid nor FRP induction of FT reduced TFL1 mRNA accumulation (Supplementary Figs. 8c and 9d).
Competition of TFL1 from FD by FT is not limited to the LFY locus. We tested whether FT induction by FRP competes TFL1 from the other direct TFL1–FD target loci we identified (Fig. 1f). FRP treatment reduced TFL1 occupancy at all loci tested (Fig. 4f). To confirm that FT indeed occupies the TFL1–FD bound sites at these loci, we also conducted ChIP-qPCR in FT-HAER after steroid induction. In the estradiol treated samples, we saw significant FT recruitment to the TFL1–FD bound regions at all loci tested (Fig. 4g). We conclude that the antagonism between FT and TFL119,21,27 relies on competition for FD bound at the chromatin of shared target loci (Fig. 4h).
Direct TFL1–FD repressed genes promote onset of flower formation and endogenous signalling
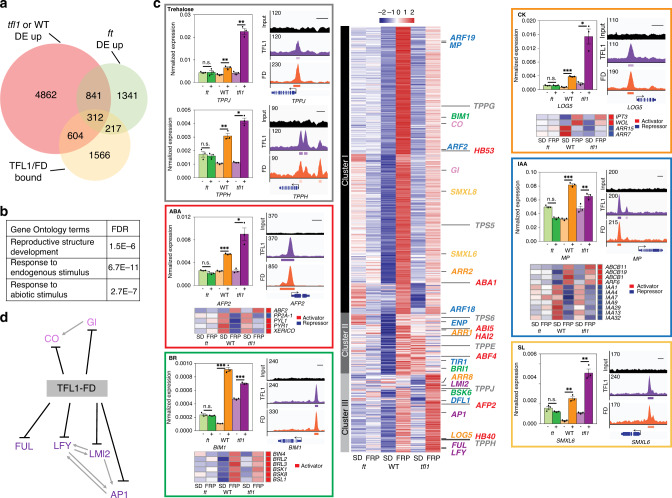
Our findings place florigens directly upstream of LFY, yet prior genetic data suggest that florigens act both upstream of and in parallel with LFY39,50. To gain insight into additional gene expression programs repressed by the TFL1–FD complex, we next conducted RNA-seq with and without FRP treatment. We isolated inflorescences with associated primordia from 42-day-old short-day-grown ft mutant, wild-type and tfl1 mutant plants and identified the significant gene expression changes in each genotype relative to untreated siblings. On the basis of Principle Component Analysis (PCA) and replicate analysis, RNA-seq quality was high (Supplementary Fig. 11). We next defined genes directly repressed by TFL1–FD. Towards this end, we focussed on TFL1–FD complex bound loci that exhibit FT-dependent de-repression upon photoinduction. Six-hundred four TFL1–FD bound genes were significantly (DESeq2 adjusted p < 0.005) de-repressed upon FRP treatment in the wild-type or in tfl1 mutants but not in ft mutants (Fig. 5a). GO term enrichment linked the TFL1–FD repressed genes to reproductive development and to response to endogenous and abiotic signals (Fig. 5b).
Fig. 5. Genes directly repressed by the TFL1–FD complex.
a Six-hundred four genes bound by TFL1 and FD are de-repressed after a single FRP specifically in the wild-type or in tfl1 mutants. b Gene ontology term enrichment (Goslim AgriGO v2) for the 604 direct TFL1–FD complex repressed genes. c K-means clustering of the direct TFL1–FD complex repressed genes (centre). Heatmap with gene names colour coded for the pathway they act in. Onset of reproduction (light purple), floral fate (purple), sugar signalling (grey), abscisic acid (ABA, red), brassinosteroid (BR, green), cytokinin (CK, orange), auxin (IAA, blue) and strigolactone (SL, yellow). In colour-coded boxes: Left: Independent confirmation of gene expression changes of direct TFL1–FD targets by qRT-PCR of plants with (+) or without (−) a single FRP. Shown are mean ± SEM of three independent biological replicates (black dots). P = values (unpaired one-tailed t-test) TPPJ: n.s. ft = 0.45, **WT = 0.0044, **tfl1 = 0.0027; TPPH: n.s. ft = 0.99, ** WT = 0.004, **tfl1 = 0.002; AFP2: n.s. ft = 0.96, ***WT = 2E−06, **tfl1 = 0.01; BIM1: n.s. ft = 0.76, ***WT = 0.0007, ***tfl1 = 1.7E−04; LOG5: n.s. ft = 0.44, ***WT = 8.7E−06, *tfl1 = 0.012; MP: n.s. ft = 0.99, ***WT = 7.2E−05, **tfl1 = 0.007; SMXL6: n.s. ft = 0.91, **WT p value = 0.003, **tfl1 = 0.005. Right: ChIP-seq-binding screenshots. Below: (where applicable) heatmaps of indirect (downstream) FT-dependent gene expression changes of known pathway activators (red in sidebar) or repressors (blue in sidebar). Indirect target genes include: abscisic acid: upregulation of some positive regulators of abscisic acid response (ABF2) and repression of others (PYL1, PYR1, XERICO)58,59; Brassinosteroid: upregulation of multiple positive signalling pathway components (BRL2, BRL3, BSK1, BSK8, BSL1 and BIN4)123. Cytokinin: upregulation of cytokinin production (IPT3) and perception (WOL) and downregulation of negative cytokinin response regulators (ARR7, ARR15)124. Auxin: upregulation a positive auxin response regulator (ARF6) and downregulation of negative auxin response regulators (IAAs)125. d Interaction network of TFL1–FD complex repressed targets linked to onset of reproduction (CO, GI) and floral fate (all others). Previously described interactions2,3,32 are indicated by grey arrows. See also Supplementary Figs. 11–14.
K-means clustering of the 604 genes identified three main patterns of gene expression. Genes encoding promoters of floral fate32 (LFY, AP1, FUL and LMI2) clustered together (cluster III in Fig. 5c) and displayed stronger upregulation in tfl1 mutants than in the wild type. This pattern of de-repression was confirmed for all four loci using independent biological samples and qRT-PCR (Supplementary Fig. 12a). SOC1 clusters with these genes, but was not included in further analyses because it was weakly, but significantly, de-repressed in ft mutants (Fig. 5c, Supplementary Data 1 and Supplementary Fig. 12b). By contrast, CO and GI, which promote cessation of vegetative development2,3, were more strongly upregulated in the wild-type than in tfl1 mutants (cluster I in Fig. 5c), perhaps because these genes are already partially de-repressed in tfl1 mutants in the absence of FRP treatment. Indeed, like RNA-seq, qRT-PCR of independent biological replicates revealed higher accumulation of GI and CO in untreated tfl1 mutant compared to wild-type plants (Fig. 5c, Supplementary Data 1 and Supplementary Fig. 12b). Cluster II genes are only upregulated in the wild type and may represent genes that are transiently de-repressed. Our data identify the TFL-FD complex as a hub for repression of key regulators of the onset of reproductive development and of the switch to flower fate (Fig. 5c)
Consistent with the GO-term enrichment analysis (Fig. 5b), combined ChIP-seq and RNA-seq analysis additionally identified components of endogenous stimulus response. We identified genes linked to sugar signalling (trehalose-6-phosphate) and hormonal signalling and response (abscisic acid, cytokinin, brassinosteroid, auxin and strigolactone) as direct TFL1–FD complex repressed targets (Fig. 5c). Using qRT-PCR and independent biological samples, we confirmed FT-dependent de-repression of members of these pathways by FRP photoinduction (Fig. 5c). Several of the identified pathways link to repression of branching or to promotion of onset of flower formation in the inflorescence. For example, we identified four trehalose-6-phosphate phosphatases (TPPH, TPPJ, TPPG and TPPE) as direct TFL1–FD complex repressed targets; TPPs were recently shown to repress branching in the maize inflorescence51. In addition, auxin and the auxin-activated transcription factor MONOPTEROS (MP) were direct TFL1–FD complex repressed targets (Fig. 5c). MP promotes the switch to floral fate in Arabidopsis52 and the tomato ortholog of TFL1 executes its role in inflorescence architecture at least in part by modulating auxin flux and response53. Finally, we identified key components of the brassinosteroid pathway including the BRI1 receptor and the bHLH transcription factor BIM154,55 as direct TFL1–FD complex repressed targets. Brassinosteroid signalling represses inflorescence branching in Setaria56. The combined data implicate TFL1–FD in direct repression of genes that promote floral fate or repress branch fate, consistent with the role of TFL1 in promoting branch formation.
We also identified the cytokinin activating enzyme LOG557, abscisic acid biosynthesis (ABA1) and response regulators (ABI5, ABF4, APF2)58,59, and components of strigolactone signalling, SMXL6 and SMXL860,61, as direct TFL1–FD complex repressed targets.
To gain further insight into the role of the TFL1–FD complex in hormone signalling, we next assessed indirect, downstream, gene expression changes triggered by FRP treatment. In particular, we identified genes not bound by TFL1 or FD that were significantly differentially expressed (DESeq2 adjusted p < 0.005) in the wild type and in tfl1, but not in ft. The identified indirect targets provide a ‘molecular phenotype’ that is consistent with de-repression of the auxin, brassinosteroid and cytokinin hormone pathways upon FRP treatment in the wild-type and in tfl1 mutants (Fig. 5c). By contrast, the abscisic acid signalling pathway signature was more complex (Fig. 5c). Our combined data uncover a prominent role for the TFL1–FD complex in regulation of endogenous signalling.
It is conceivable that components of some of the identified TFL1–FD dependant pathways (strigolactone, cytokinin, auxin, abscisic acid as well as sugar signalling) may modulate additional aspects of the inflorescence architecture, such as branch outgrowth62–64. Support for this hypothesis comes from our phenotypic analyses. We examined the effect of a single FRP on inflorescence architecture in the ft mutant, the wild-type and the tfl1 mutant. Photoperiod induction triggered a reduction in the number of branches, but not cauline leaves, formed in the wild type and more strongly, in tfl1 (Supplementary Fig. 13a–j). This suggests that branch meristems adopt floral fate upon stimulus perception65. tfl1 mutants also formed fewer branches than the wild type in the absence of photoperiod. These phenotypes are consistent with the observed gene expression changes (Fig. 5c). In addition, FRP triggered a significant increase in inflorescence branch outgrowth in both wild-type and tfl1 plants (Supplementary Fig. 13k). FRP had no phenotypic effect in ft mutants. Our combined data suggest that florigens tune plant form to the environment by controlling expression of master developmental regulators and endogenous signalling pathway components. These developmental changes likely require large-scale transcriptional reprograming in the context of chromatin. Consistently, we identified transcriptional co-regulators and chromatin regulators among the direct TFL1–FD repressed targets (Supplementary Fig. 14a, b).
Discussion
Here we identify LFY, a master regulator of flower fate33,34, as a target under dual opposite transcriptional regulation by TFL1 and FT and demonstrate that FT activation of LFY expression is critical to promote floral fate. We provide a molecular framework for the antagonistic roles5,27 of FT and TFL1 that relies on competition for bZIP transcription factor mediated access to binding sites at regulatory regions of shared target loci. Additional support for this mechanism comes from recent in vitro studies21.
Our data suggest that TFL1 may not simply prevent access of the FT co-activator to the chromatin23 but may be an active repressor, as mutating bZIP-binding sites results in LFY de-repression specifically in the TFL1 expression domain. The identity of the transcription factors that activate LFY in the centre of the inflorescence shoot apex in the absence of PEBP/FD binding to LFY is not known. Our identification of FT recruiting motifs in the second exon of LFY fits with prior data demonstrating that the 2.3 kb upstream intergenic ‘LFY promoter’ is unresponsive to FT38. This upstream regulatory region drives reporter expression in similar domains as endogenous LFY66. The requirement of the bZIP motifs for LFY upregulation in the context of the genomic construct, which contains the 2.3 kb ‘LFY promoter’, suggests the presence of repressive regulatory elements in the genic region of LFY.
We identify hundreds of TFL1–FD repressed genes many of which, based on our computational analyses of recently published FD and TFL1 ChIP-seq datasets17,30, are also immediate early gTFL1 or gFD targets in long-day conditions. Eighty two percent of our 604 high-confidence TFL1–FD repressed genes are present in at least one of the long-day ChIP-seq datasets (Supplementary Fig. 3b). The 604 direct TFL1–FD repressed genes include key regulators of onset of the reproductive phase and of floral fate. Of note, TFL1 opposes not only LFY, but also LFY targets, such as LMI2 and AP167,68. This is consistent with prior genetic investigations that place TFL1 both upstream of LFY and as a modulator of plant response to LFY50. Finally, we link the TFL1–FD complex to repression of diverse endogenous signalling pathways including sugar and hormonal signals. Several of these pathways have been shown to impact the switch from branch or flower fate in other plant species51,53,56. The combined data point to an important role of the hormonal environment for the switch from branch to flower fate in primordia of the inflorescence. Our findings also set the stage for elucidating communalities as well as differences between florigen regulated cell fate reprogramming during flower initiation and other developmental pathways under seasonal control by florigens such as tuberization, bulb formation and seed dormancy69–72.
Changes in the relative balance of activating and repressive PEBP family members occurred during domestication of diverse crop species to give rise to desirable traits like everbearing and compact growth habits5,8,69,73. Thus, mechanistic insight into the antagonism and identification of the targets of PEBPs will benefit traditional or genome editing-based crop improvement. It should further facilitate elucidation that how PEBP protein act as co-activators or co-repressors in the nucleus.
Methods
Plant materials
Arabidopsis ecotype Columbia plants were grown in soil at 22 °C in long-day photoperiod (LD, 16 h light/8 h dark, 100 µmol/m2 s) or short-day photoperiod (SD, 8 h light/16 h dark, 120 µmol/m2 s). gFD-GUS20, fd-1 null mutants74, tfl1-14 hypomorph mutant27,75, tfl1-1 severe mutant27,75,76, lfy-1 null mutant33,77,78, ft-10 null mutant79, 35S:LFY68 and 35S:TFL1 (ref. 35) were previously described. 35S:LFY (Landsberg erecta) was introgressed into the Columbia background through backcrossing. gFD-GUS80 was crossed into the fd-1 null mutant background.
Constructs for transgenic plants
For gTFL1-GFP, GFP followed by a peptide linker (GGGLQ) was fused to an 8.4 kb BamHI (NEB, R0136S) genomic fragment from lambda TFG4 (ref. 81). This fragment was introduced into the binary vector pCGN1547 (ref. 82). For TFL1ER and FT-HAER, TFL1 and FT were PCR amplified from cDNA; in the case of FT, the 3′ primer contained three times Hemagglutinin (HA) plus a stop codon. PCR products were cloned into pENTRD-TOPO (Invitrogen, K243520) and shuffled into pMDC7 (ref. 83) by LR reaction (Invitrogen, 11791-020).
For LFY-GUS reporters, the bacterial beta-glucuronidase (GUS) gene from the pGWB3 (ref. 84) binary vector was fused with pENTRD-TOPO vector containing the 2290-bp LFY promoter66 alone (pLFY:GUS), the LFY promoter and LFY genic region up to and including the first intron (pLFYi1:GUS), or the LFY promoter and LFY genic region up to and including the second LFY intron (pLFYi2:GUS) by LR reaction. bZIP-binding site mutations in LFY e2 (pLFYi2m3:GUS) were generated by Ω-PCR85. gGLFY was constructed by PCR amplifying a 4929-bp genomic LFY fragment (gLFY), including the 2290-bp LFY promoter, from genomic DNA followed by cloning into the KpnI-HF (NEB, R3124S) and NotI-HF (NEB, R3189S) digested pENTR3C vector by Gibson Assembly. Next, GFP was inserted at position + 94 bp, as previously described for pLFY:GLFY42,80, by Ω-PCR85. bZIP-binding site mutations were introduced into pENTR3C-gGLFY by Ω-PCR to generate gGLFYm3. Both constructs were shuffled into pMCS:GW86 using LR reaction. To create gLFY:GUS, the 4929-bp genomic LFY clone minus the stop codon and the GUS fragment were PCR amplified and inserted into linearized pENTR3C by Gibson Assembly. For gLFYm3:GUS, bZIP-binding site mutations were introduced into pENTR3C-gLFY:GUS by Ω-PCR. To test recruitment of TFL1 and FT to e2 of LFY, wild-type (e2) or bZIP-binding site mutated exon 2 (e2m3) were PCR amplified and cloned into pGWB3 (ref. 84).
For test of recruitment, LFY e2 and e2m3 were amplified by forward (5′-caccAACAGCAGCAGAGACGGAGAAAGAA-3′) and reverse (5′-TCGTACAAGTGGAACAGATAATC-3′) primers and cloned into pGWB3 binary vectors, which were transformed into gTFL1-GFP and 35S:FT-HAER.
For pFT4kb:amiRFT, a 3994-bp truncated FT promoter87 was PCR amplified from genomic DNA as was the published amiRFT44 from pRS300 (ref. 88). The amiRFT fragment was introduced into EcoRI-HF (NEB, R3101S) digested pENTR3C (Thermo Fisher Scientific, A10464) by Gibson Assembly (NEB, E5510S) and shuffled into binary vector pMCS:GW86 using LR reaction, which resulted in pMCS:amiRFT. The previously described 3994-bp FT promoter was inserted to XhoI (NEB, R0146S) digested pMCS:amiRFT by Gibson Assembly.
Genomic DNA was extracted using the GenElute Plant Genomic DNA Miniprep Kit (Sigma-Aldrich, G2N70). Primer sequences are listed in Supplementary Table 2. All constructs were sequence verified prior to transformation into plants with Agrobacterium strain GV3101 by floral dip89. Plant lines generated are listed in Supplementary Table 3.
Imaging
Images were taken with a Canon EOS Rebel T5 camera for plant phenotypes and yeast one-hybrid assays, or with a stereo microscope (Olympus SZX12) equipped with a colour camera (Olympus LC30) for GUS images and inflorescence phenotypes. For GFP images, a Leica TCS SP8 Multiphoton Confocal with a 20× objective was used with a 488 nm excitation laser and emission spectrum between 520 and 550 nm (GFP) or 650–700 nm (chlorophyll autofluorescence) using standard imaging techniques90,91. For tfl1-1 gTFL1-GFP in short-day photoperiod, shoot apices were sectioned longitudinally on an oscillating tissue slicer (Electron Microscopy Sciences, OTS-4000) after embedding in 5% Agar (Fisher Scientific, DF0812-07-1).
Plant treatment and gene expression analysis
For test of gene expression, 16-day-old TFL1ER or 12-day-old FT-HAER plants grown in LD were induced by a single spray application of 10 μmol beta-estradiol (Sigma-Aldrich, 8875-250MG) dissolved in DMSO (Fisher Scientific, BP231-1L) and 0.015% Silwet L-77 (PlantMedia, 30630216-3). Mock solution consisted of 0.1% DMSO and 0.015% Silwet. To probe FT recruitment to and TFL1 occupancy at the LFY locus, 42-day-old FT-HAER gTFL1-GFP plants grown in SD were treated by a single spray application of 10 μmol beta-estradiol or mock solution. In all cases, tissues were harvested 4 h after treatment. To test for gain-of-function phenotypes in long-day photoperiod, FT-HAER, TFL1ER and FT-HAER gTFL1-GFP plants were treated with 10 μmol beta-estradiol or mock solution from 5-day onwards every other day until bolting.
FRP was applied at the end of the short day (ZT8) for 24 h using a Percival Scientific E30LED45 with red (660 nm) to far-red (730 nm) ratio = 0.5 and light intensity 80 µmol/m2 s. Control plants were kept in regular short-day conditions (16 h dark and 8 h light, red to far-red ratio = 12 and 120 µmol/m2 s light intensity) for 24 h. Light intensity and spectral composition were measured by an Analytical Spectral Devices FieldSpec Pro spectrophotometer.
For qRT-PCR analysis, total RNA was extracted from leaves or shoot apices using TRIzol (Thermo Fisher Scientific) and purified with the RNeasy Mini Kit (Qiagen, 74104). cDNA was synthesized using SuperScript III First-Strand Synthesis (Invitrogen, 18080051) from 1 μg of RNA. Real time PCR was conducted using a cDNA standard curve. Normalized expression levels were calculated using the 2ˆ(−delta delta CT) method with the housekeeping gene UBQ10 (AT4G05320) as the control. Where expression of multiple different genes was compared, normalized gene expression is shown relative to the control treatment. Primer sequences are listed in Supplementary Table 2.
Yeast one-hybrid assay
LFY e2 and the bZIP-binding site mutated version (e2m3) were cloned into the KpnI-HF and XhoI linearized pAbAi vector (Takara) by Gibson Assembly and integrated into the yeast genome following the Matchmaker Gold Yeast One-Hybrid protocol (Takara) and the Y1Golden strain (Takara). Coding sequences of FD and TFL1 were cloned into the pENTRD-TOPO vector. After sequencing, constructs were shuffled into either pDEST32 or pDEST22 (Takara) by LR reaction and transformed into the DNA-binding region containing yeast strain. Empty pDEST32 and pDEST22 served as negative controls. Growth was assayed after serial dilution on growth media with or without 60 ng/ml Aureobasidin A (Clontech, 630499). Primer sequences are listed in Supplementary Table 2.
ChIP-qPCR, ChIP-seq and data analysis
Forty two-day-old short-day grown plants were trimmed and 1.6 g of non-bolted inflorescences were harvested from 36 plants. Chromatin immunoprecipitation was conducted following a published protocol92 for ChIP-qPCR. For ChIP-seq, each biological replicate consisted of eight individual IP reactions pooled into one MinElute PCR (Qiagen, 28004) purification column. For ChIP-seq and ChIP-qPCR, anti-GFP antibody (Thermo Fisher Scientific, A-11122; 1:200 dilution) and anti-GUS antibody (Abcam, ab50148; 1:200 dilution) were used. The antibodies were validated by the manufacturers. ChIP-qPCR was performed using Platinum Taq DNA Polymerase (Invitrogen, 10966034) and EvaGreen dye (Biotium, 31000). For ChIP-qPCR, the value of the ChIP samples was normalized over that of input DNA as previously described92. Non-transgenic wild-type plants were used as the negative genetic control for anti-GFP and anti-GUS antibody ChIP. The TA3 retrotransposon (AT1G37110) was used as the negative control region for ChIP-qPCR. Primer sequences are listed in Supplementary Table 2.
Anti-GFP ChIP-seq was performed for gTFL1-GFP (A), gTFL1-GFP (B), fd-1 gTFL1-GFP and control samples (non-epitope containing plants). Anti-GUS ChIP-seq was performed for gFD-GUS. Two biological replicates were sequenced in each case. Dual index libraries were prepared for the ten ChIP samples listed above and for four input samples using the SMARTer ThruPLEX DNA-Seq Kit (Takara Bio, R400406). Library quantification was performed with the NEBNext Library Quant Kit for Illumina (NEB, E7630). Single-end sequencing was conducted using High Output Kit v2.5 (Illumina, TG-160-2005) on the NextSeq 500 platform (Illumina).
FastQC v0.11.5 was performed on both the raw and trimmed93 reads using TRIMMOMATIC v0.3693 (ILLUMINACLIP:adaptors.fasta:2:30:10 LEADING:3 TRAILING:3 MINLEN:50) to confirm sequencing quality94. Reads with MAPQ ≥ 30 (SAMtools v1.7)95 uniquely mapping to the Arabidopsis Information Resource version 10 (TAIR 10)96 that were not flagged as PCR or optical duplicates by Bowtie2 v2.3.1(ref. 97,98) were analyzed further by principal component analysis (PCA) using the plotPCA function of deepTools99. Reads were further processed following ENCODE guidelines100, followed by cross-correlation analysis with the predict function of MACS2 (ref. 101) to empirically determine the fragment length. Significant ChIP peaks and summits (summit q value ≤ 10−10) were identified in MACS2 for the pooled ChIP relative to the pooled negative controls (ChIP in non-transgenic wild type). Peak overlap (≥1 bp) was computed using BEDTools intersect v2.26.0 (ref. 102) and statistical significance was computed using the hypergeometric test assuming a ‘universe’ of 10,000 possible peaks103. Heatmaps were generated using deepTools v3.1.2 (ref. 99). The 3308 TFL1 and 4422 FD peaks were mapped to 3699 and 4493 Araport11 (ref. 104) annotated genes, respectively, if the peak summit was intragenic or located ≤4 kb upstream of the transcription start site. Recently published datasets were analyzed in identical fashion. Genomic distribution of peak summits was called using the ChIPpeakAnno library104,105.
De novo motif analysis was conducted using MEME-ChIP v5.0.2 (Discriminative Mode)106 and HOMER v4.10 (ref. 107) for MACS2 q value ≤ 10−10 peak summits (±250 base pairs) compared to genome-matched background (unbound regions from similar genomic locations as the peak summits) as previously described108,109.
GO term enrichment analyses were performed using GOSlim in agriGO v2.0 (ref. 110) and significantly enriched GO terms with q value < 0.0001 (FDR, Benjamini and Yekutieli method111) were identified.
Correlation analyses
Public ChIP-seq datasets for FD30, for TFL117, LFY112 and an unpublished LFY ChIP-seq dataset from our lab (GEO accession GSE141706) were analyzed as described above for TFL1 and FD ChIP-seq. To compare the relationship between all ChIP-seq datasets we calculated Pearson correlation coefficients for reads in regions of interest using deeptools v3.1.299. Regions of interest were comprised of the combined significant peak regions (MACS2 ≤ q value 10−10) of all ChIP-seq datasets and read signal was derived from the sequencing-depth normalized bigwig file for each sample.
RNA-seq and data analysis
A single 24 h FRP was applied to 42-day-old short-day grown ft-10 mutants, wild-type and tfl1-1 mutants, starting at the end of the day (ZT8). After the treatment, 0.1 g of inflorescence shoots were harvested for each biological replicate after removing all leaves and roots. Three biological replicates were prepared for each experiment. RNA quantity and quality were analyzed by Qubit BR assay (Thermo Fisher Scientific, Q10210) and Agilent RNA 6000 Nano Kit (Agilent, 5067-1511) on an Agilent 2100 bioanalyzer, respectively. Libraries were constructed from 1 µg total RNA using the TruSeq RNA Sample Prep Kit (Illumina, RS-122-2001). After library quantification with the NEBNext Library Quant Kit for Illumina (NEB, E7630), single-end sequencing was conducted using the NextSeq 500 platform (Illumina).
RNA-seq analysis was conducted using FastQC v0.11.5 (ref. 94) on raw sequences before and after trimming using Trimmomatic v0.36 (ref. 93) (ILLUMINACLIP:adapters.fasta:2:30:10 LEADING:3 TRAILING:3 MINLEN:50) to confirm sequencing quality94. Reads were mapped using the STAR mapping algorithm113 (–sjdbOverhang 100 --outSAMprimaryFlag AllBestScore --outSJfilterCountTotalMin 10 5 5 5 --outSAMstrandField intronMotif --outFilterIntronMotifs RemoveNoncanonical --alignIntronMin 60 --alignIntronMax 6000 --outFilterMismatchNmax 2), to the TAIR 10 Arabidopsis genome-assembly96, and Araport11 Arabidopsis genome-annotation104. Specific read coverage was assessed with HT-Seq114 (--stranded = ‘no’ -minaqual = 30).
For PCA, raw read counts were subjected to variance stabilizing transformation and projected into two principal components with the highest variance115–117. In parallel, raw reads were adjusted for library size by DESeq2 v1.24.0 (ref. 118). After PCA, two biological replicates per genotype and treatment were selected for further analysis. Gene normalized z-scores were used for k-means (MacQueen) clustering119. Pairwise differential expression analyses were performed by comparing FRP and untreated pooled normalized read counts in each genotype using default DESeq2 parameters with no shrinkage (ref. 118) and an adjusted p value cut-off ≤ 0.005.
Photoperiod shift phenotype analysis
A single 24-h far-red light enriched photoperiod shift (FRP) was applied to 42-day-old short-day grown ft-10 mutants, wild-type and tfl1-1 mutants as for RNA-seq, followed by further growth in short-day conditions. To asses onset of reproductive development, the number of rosette leaves formed were counted at bolting. To analyze the inflorescence architecture, the number of sessile buds, outgrowing branches, flower branches, and single flowers subtended by a cauline leaf were counted weekly after bolting until the first normal flower (not subtended by a cauline leaf) formed.
Statistical analyses
The Kolmogorov–Smirnov (K–S) test120 was used to assess whether the data were normally distributed. All ChIP and qRT-PCR data were normally distributed. An unpaired one-tailed t-test was used to test for changes in one direction. Error bars represent the standard error of the mean (SEM). Two to three independent biological replicates were analyzed. For multiple-group comparisons (phenotypes) the non-parametric Kruskal–Wallis test121 followed by the Dunn’s post hoc test122 were employed. Box and whisker plots display minima and maxima (whiskers), lower and upper quartile (box) and median (red vertical line).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Descriptions of Additional Supplementary Files
Acknowledgements
We thank undergraduate students Gabriela M. Blandino, Xindi Chen and Zubaida Salman and Dietrich James Nigh for help with the experiments, Dr. John D. Wagner for input on the manuscript and the Plant Biology group as well as Wagner lab members for feedback on this project. This research was funded by National Science Foundation IOS grant 1557529 and 1905062 to D.W.
Source data
Author contributions
D.W. and Y.Z. conceived of the study and Y.Z. conducted the majority of the experiments. S.K. conducted the bioinformatic analyses. N.Y. and C.W.J. identified the optimal stage to study primordium fate regulation by TFL1, conducted initial TFL1 ChIP analyses and mapped the FD-binding sites in LFY. R.J. and Y.Z. constructed and sequenced ChIP-seq libraries, K.G generated the biologically active genomic GFP-TFL1 construct. D.W. wrote the manuscript with the help of Y.Z. and input from all other authors.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper, its Supplementary information files and public data repositories. Source data are provided with this paper. The ChIP-seq and RNA-seq datasets were deposited to the GEO database (GSE141894). Individual replicates and P values for all figures are provided as a source data file. Source data are provided with this paper.
Code availability
Scripts for peak to gene annotation can be found at https://github.com/sklasfeld/ChIP_Annotation.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Cheol Woong Jeong, Nobutoshi Yamaguchi.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-18782-1.
References
- 1.Song J, Irwin J, Dean C. Remembering the prolonged cold of winter. Curr. Biol. 2013;23:R807–R811. doi: 10.1016/j.cub.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T. Photoperiodic flowering: time measurement mechanisms in leaves. Annu. Rev. Plant Biol. 2015;66:441–464. doi: 10.1146/annurev-arplant-043014-115555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrés F, Coupland G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012;13:627. doi: 10.1038/nrg3291. [DOI] [PubMed] [Google Scholar]
- 4.Teo ZW, Song S, Wang YQ, Liu J, Yu H. New insights into the regulation of inflorescence architecture. Trends Plant Sci. 2014;19:158–165. doi: 10.1016/j.tplants.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Périlleux C, Bouché F, Randoux M, Orman-Ligeza B. Turning meristems into fortresses. Trends Plant Sci. 2019;24:431–442. doi: 10.1016/j.tplants.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Prusinkiewicz P, Erasmus Y, Lane B, Harder LD, Coen E. Evolution and development of inflorescence architectures. Science. 2007;316:1452–1456. doi: 10.1126/science.1140429. [DOI] [PubMed] [Google Scholar]
- 7.Park SJ, et al. Optimization of crop productivity in tomato using induced mutations in the florigen pathway. Nat. Genet. 2014;46:1337–1342. doi: 10.1038/ng.3131. [DOI] [PubMed] [Google Scholar]
- 8.Eshed, Y. & Lippman, Z. B. Revolutions in agriculture chart a course for targeted breeding of old and new crops. Science366, eaax0025 (2019). [DOI] [PubMed]
- 9.Lifschitz E, Ayre BG, Eshed Y. Florigen and anti-florigen—a systemic mechanism for coordinating growth and termination in flowering plants. Front. Plant Sci. 2014;5:465. doi: 10.3389/fpls.2014.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlgren A, et al. Evolution of the PEBP gene family in plants: functional diversification in seed plant evolution. Plant Physiol. 2011;156:1967–1977. doi: 10.1104/pp.111.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kardailsky I, et al. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- 13.Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. Inflorescence Commitment and Architecture in Arabidopsis. Science. 1997;275:80–83. doi: 10.1126/science.275.5296.80. [DOI] [PubMed] [Google Scholar]
- 14.Conti L, Bradley D. TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell. 2007;19:767–778. doi: 10.1105/tpc.106.049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathieu J, Warthmann N, Kuttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr. Biol. 2007;17:1055–1060. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr. Biol. 2007;17:1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Goretti D, et al. TERMINAL FLOWER1 functions as a mobile transcriptional cofactor in the shoot apical meristem. Plant Physiol. 2020;182:2081–2095. doi: 10.1104/pp.19.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbesier L, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 19.Taoka K, et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature. 2011;476:332–335. doi: 10.1038/nature10272. [DOI] [PubMed] [Google Scholar]
- 20.Abe M, et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko-Suzuki M, et al. TFL1-like proteins in rice antagonize rice FT-like protein in inflorescence development by competition for complex formation with 14-3-3 and FD. Plant Cell Physiol. 2018;59:458–468. doi: 10.1093/pcp/pcy021. [DOI] [PubMed] [Google Scholar]
- 22.Wigge PA, et al. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- 23.Ho WW, Weigel D. Structural features determining flower-promoting activity of Arabidopsis FLOWERING LOCUS T. Plant Cell. 2014;26:552–564. doi: 10.1105/tpc.113.115220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn JH, et al. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 2006;25:605–614. doi: 10.1038/sj.emboj.7600950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanzawa Y, Money T, Bradley D. A single amino acid converts a repressor to an activator of flowering. Proc. Natl Acad. Sci. USA. 2005;102:7748–7753. doi: 10.1073/pnas.0500932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pin PA, et al. An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science. 2010;330:1397–1400. doi: 10.1126/science.1197004. [DOI] [PubMed] [Google Scholar]
- 27.Hanano S, Goto K. Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell. 2011;23:3172–3184. doi: 10.1105/tpc.111.088641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohn EJ, et al. The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proc. Natl Acad. Sci. USA. 2007;104:18801–18806. doi: 10.1073/pnas.0708236104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abelenda JA, et al. Source-sink regulation is mediated by interaction of an FT homolog with a SWEET protein in potato. Curr. Biol. 2019;29:1178–1186 e6. doi: 10.1016/j.cub.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Collani S, Neumann M, Yant L, Schmid M. FT modulates genome-wide DNA-binding of the bZIP transcription factor FD. Plant Physiol. 2019;180:367–380. doi: 10.1104/pp.18.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fornara, F., de Montaigu, A. & Coupland, G. SnapShot: control of flowering in Arabidopsis. Cell141, 550, 550.e1–e2 (2010). [DOI] [PubMed]
- 32.Wagner D. Key developmental transitions during flower morphogenesis and their regulation. Curr. Opin. Genet Dev. 2017;45:44–50. doi: 10.1016/j.gde.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM. LEAFY controls floral meristem identity in Arabidopsis. Cell. 1992;69:843–859. doi: 10.1016/0092-8674(92)90295-N. [DOI] [PubMed] [Google Scholar]
- 34.Weigel D, Nilsson O. A developmental switch sufficient for flower initiation in diverse plants. Nature. 1995;377:495–500. doi: 10.1038/377495a0. [DOI] [PubMed] [Google Scholar]
- 35.Ratcliffe OJ, et al. A common mechanism controls the life cycle and architecture of plants. Development. 1998;125:1609–1615. doi: 10.1242/dev.125.9.1609. [DOI] [PubMed] [Google Scholar]
- 36.Stergachis AB, et al. Exonic transcription factor binding directs codon choice and affects protein evolution. Science. 2013;342:1367–1372. doi: 10.1126/science.1243490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reyna-Llorens I, et al. Ancient duons may underpin spatial patterning of gene expression in C4 leaves. Proc. Natl Acad. Sci. USA. 2018;115:1931–1936. doi: 10.1073/pnas.1720576115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blazquez MA, Weigel D. Integration of floral inductive signals in Arabidopsis. Nature. 2000;404:889–892. doi: 10.1038/35009125. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Garcia L, et al. Different roles of flowering-time genes in the activation of floral initiation genes in Arabidopsis. Plant Cell. 1997;9:1921–1934. doi: 10.1105/tpc.9.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon J, Lee H, Kim M, Lee I. Analysis of flowering pathway integrators in Arabidopsis. Plant Cell Physiol. 2005;46:292–299. doi: 10.1093/pcp/pci024. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Oh M, Park H, Lee I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. Plant J. 2008;55:832–843. doi: 10.1111/j.1365-313X.2008.03552.x. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi N, et al. Gibberellin acts positively then negatively to control onset of flower formation in Arabidopsis. Science. 2014;344:638–641. doi: 10.1126/science.1250498. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Farrona S, Klemme S, Turck FK. Post-fertilization expression of FLOWERING LOCUS T suppresses reproductive reversion. Front. Plant Sci. 2014;5:164. doi: 10.3389/fpls.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly specific gene silencing by artificial MicroRNAs in Arabidopsis. Plant Cell. 2006;18:1121–1133. doi: 10.1105/tpc.105.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hempel FD, et al. Floral determination and expression of floral regulatory genes in Arabidopsis. Development. 1997;124:3845–3853. doi: 10.1242/dev.124.19.3845. [DOI] [PubMed] [Google Scholar]
- 46.Valverde F, et al. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi N, et al. A molecular framework for auxin-mediated initiation of flower primordia. Dev. Cell. 2013;24:271–282. doi: 10.1016/j.devcel.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi A, et al. The MicroRNA-regulated SBP-box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Developmental Cell. 2009;17:268–278. doi: 10.1016/j.devcel.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee C, et al. Genetic interactions reveal the antagonistic roles of FT/TSF and TFL1 in the determination of inflorescence meristem identity in Arabidopsis. Plant J. 2019;99:452–464. doi: 10.1111/tpj.14335. [DOI] [PubMed] [Google Scholar]
- 50.Ratcliffe OJ, Bradley DJ, Coen ES. Separation of shoot and floral identity in Arabidopsis. Development. 1999;126:1109–1120. doi: 10.1242/dev.126.6.1109. [DOI] [PubMed] [Google Scholar]
- 51.Claeys H, et al. Control of meristem determinacy by trehalose 6-phosphate phosphatases is uncoupled from enzymatic activity. Nat. Plants. 2019;5:352–357. doi: 10.1038/s41477-019-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaguchi N, Wu MF, Winter C, Wagner D. LEAFY together with polar auxin transport coordinates Arabidopsis flower development. Plants. 2014;3:251–265. doi: 10.3390/plants3020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva WB, et al. SELF-PRUNING acts synergistically with DIAGEOTROPICA to guide auxin responses and proper growth form. Plant Physiol. 2018;176:2904–2916. doi: 10.1104/pp.18.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lozano-Elena F, Cano-Delgado AI. Emerging roles of vascular brassinosteroid receptors of the BRI1-like family. Curr. Opin. Plant Biol. 2019;51:105–113. doi: 10.1016/j.pbi.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Yin Y, et al. A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell. 2005;120:249–259. doi: 10.1016/j.cell.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, et al. Brassinosteroids modulate meristem fate and differentiation of unique inflorescence morphology in Setaria viridis. Plant Cell. 2018;30:48–66. doi: 10.1105/tpc.17.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuroha T, et al. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell. 2009;21:3152–3169. doi: 10.1105/tpc.109.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qin F, Shinozaki K, Yamaguchi-Shinozaki K. Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol. 2011;52:1569–1582. doi: 10.1093/pcp/pcr106. [DOI] [PubMed] [Google Scholar]
- 59.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, et al. Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-Like SMXL repressor proteins for ubiquitination and degradation. Plant Cell. 2015;27:3128–3142. doi: 10.1105/tpc.15.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soundappan I, et al. SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell. 2015;27:3143–3159. doi: 10.1105/tpc.15.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu, Y. & Wagner, D. Plant inflorescence architecture: the formation, activity, and fate of axillary meristems. Cold Spring Harb. Perspect. Biol. 12, a034652 (2019). [DOI] [PMC free article] [PubMed]
- 63.Barbier FF, Dun EA, Kerr SC, Chabikwa TG, Beveridge CA. An update on the signals controlling shoot branching. Trends Plant Sci. 2019;24:220–236. doi: 10.1016/j.tplants.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Hiraoka K, Yamaguchi A, Abe M, Araki T. The florigen genes FT and TSF modulate lateral shoot outgrowth in Arabidopsis thaliana. Plant Cell Physiol. 2013;54:352–368. doi: 10.1093/pcp/pcs168. [DOI] [PubMed] [Google Scholar]
- 65.Hempel FD, Zambryski PC, Feldman LJ. Photoinduction of flower identity in vegetatively biased primordia. Plant Cell. 1998;10:1663–1676. doi: 10.1105/tpc.10.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blazquez MA, Soowal LN, Lee I, Weigel D. LEAFY expression and flower initiation in Arabidopsis. Development. 1997;124:3835–3844. doi: 10.1242/dev.124.19.3835. [DOI] [PubMed] [Google Scholar]
- 67.Pastore JJ, et al. LATE MERISTEM IDENTITY2 acts together with LEAFY to activate APETALA1. Development. 2011;138:3189–3198. doi: 10.1242/dev.063073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagner D, Sablowski RWM, Meyerowitz EM. Transcriptional activation of APETALA1 by LEAFY. Science. 1999;285:582–584. doi: 10.1126/science.285.5427.582. [DOI] [PubMed] [Google Scholar]
- 69.McGarry RC, Ayre BG. Manipulating plant architecture with members of the CETS gene family. Plant Sci. 2012;188-189:71–81. doi: 10.1016/j.plantsci.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Lee R, Baldwin S, Kenel F, McCallum J, Macknight R. FLOWERING LOCUS T genes control onion bulb formation and flowering. Nat. Commun. 2013;4:2884. doi: 10.1038/ncomms3884. [DOI] [PubMed] [Google Scholar]
- 71.Navarro C, et al. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature. 2011;478:119–122. doi: 10.1038/nature10431. [DOI] [PubMed] [Google Scholar]
- 72.Chen M, Penfield S. Feedback regulation of COOLAIR expression controls seed dormancy and flowering time. Science. 2018;360:1014–1017. doi: 10.1126/science.aar7361. [DOI] [PubMed] [Google Scholar]
- 73.Blackman BK, Strasburg JL, Raduski AR, Michaels SD, Rieseberg LH. The role of recently derived FT paralogs in sunflower domestication. Curr. Biol. 2010;20:629–635. doi: 10.1016/j.cub.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koornneef M, Hanhart C, Van der Veen J. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- 75.Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. Inflorescence commitment and architecture in Arabidopsis. Science. 1997;275:80–83. doi: 10.1126/science.275.5296.80. [DOI] [PubMed] [Google Scholar]
- 76.Shannon S, Meeks-Wagner DR. A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell. 1991;3:877–892. doi: 10.2307/3869152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schultz EA, Haughn GW. LEAFY, a homeotic gene that regulates inflorescence development in Arabidopsis. Plant Cell. 1991;3:771–781. doi: 10.2307/3869271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haughn GW, Somerville CR. Genetic control of morphogenesis in Arabidopsis. Developmental Genet. 1988;9:73–89. doi: 10.1002/dvg.1020090202. [DOI] [Google Scholar]
- 79.Yoo SK, et al. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol. 2005;139:770–778. doi: 10.1104/pp.105.066928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu X, et al. Modes of intercellular transcription factor movement in the Arabidopsis apex. Development. 2003;130:3735–3745. doi: 10.1242/dev.00577. [DOI] [PubMed] [Google Scholar]
- 81.Ohshima S, Murata M, Sakamoto W, Ogura Y, Motoyoshi F. Cloning and molecular analysis of the Arabidopsis gene terminal flower 1. Mol. Gen. Genet. 1997;254:186–194. doi: 10.1007/s004380050407. [DOI] [PubMed] [Google Scholar]
- 82.McBride KE, Summerfelt KR. Improved binary vectors for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 1990;14:269–276. doi: 10.1007/BF00018567. [DOI] [PubMed] [Google Scholar]
- 83.Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003;133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakagawa T, Ishiguro S, Kimura T. Gateway vectors for plant transformation. Plant Biotechnol. 2009;26:275–284. doi: 10.5511/plantbiotechnology.26.275. [DOI] [Google Scholar]
- 85.Liu Y-G, Wang X, Wang F, Chen L. Robust one-Tube Ω-PCR strategy accelerates precise sequence modification of plasmids for functional genomics. Plant Cell Physiol. 2013;54:634–642. doi: 10.1093/pcp/pct047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michniewicz M, Frick EM, Strader LC. Gateway-compatible tissue-specific vectors for plant transformation. BMC Res. Notes. 2015;8:63. doi: 10.1186/s13104-015-1010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adrian J, et al. cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell. 2010;22:1425–1440. doi: 10.1105/tpc.110.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ossowski S, Schwab R, Weigel D. Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J. 2008;53:674–690. doi: 10.1111/j.1365-313X.2007.03328.x. [DOI] [PubMed] [Google Scholar]
- 89.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium -mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 90.Vitha, S., Beneš, K., Phillips, J. P. & Gartland, K. M. A. Histochemical GUS analysis. in Agrobacterium Protocols (eds. Gartland, K. M. A. & Davey, M. R.) 185–193 (Springer New York, Totowa, NJ, 1995). [DOI] [PubMed]
- 91.Prunet N, Jack TP, Meyerowitz EM. Live confocal imaging of Arabidopsis flower buds. Developmental Biol. 2016;419:114–120. doi: 10.1016/j.ydbio.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamaguchi, N. et al. PROTOCOLS: Chromatin immunoprecipitation from Arabidopsis tissues. Arabidopsis Book12, e0170 (2014). [DOI] [PMC free article] [PubMed]
- 93.Bolger AM, Usadel B, Lohse M. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data (Babraham Bioinformatics, Babraham Institute, Cambridge, 2010).
- 95.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lamesch P, et al. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 2011;40:D1202–D1210. doi: 10.1093/nar/gkr1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berardini TZ, et al. The Arabidopsis Information Resource: Making and mining the “gold standard” annotated reference plant genome. Genesis. 2015;53:474–485. doi: 10.1002/dvg.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richter AS, et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–W165. doi: 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Landt SG, et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 2012;22:1813–1831. doi: 10.1101/gr.136184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song L, et al. A transcription factor hierarchy defines an environmental stress response network. Science. 2016;354:aag1550. doi: 10.1126/science.aag1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng C-Y, et al. Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 2017;89:789–804. doi: 10.1111/tpj.13415. [DOI] [PubMed] [Google Scholar]
- 105.Zhu LJ, et al. ChIPpeakAnno: a Bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinform. 2010;11:237. doi: 10.1186/1471-2105-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Machanick P, Bailey TL. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics. 2011;27:1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiao J, et al. Cis and trans determinants of epigenetic silencing by polycomb repressive complex 2 in Arabidopsis. Nat. Genet. 2017;49:1546–1552. doi: 10.1038/ng.3937. [DOI] [PubMed] [Google Scholar]
- 109.Winter CaraM, et al. LEAFY target genes reveal floral regulatory logic, cis motifs, and a link to biotic stimulus response. Developmental Cell. 2011;20:430–443. doi: 10.1016/j.devcel.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 110.Yan H, et al. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017;45:W122–W129. doi: 10.1093/nar/gkx382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001;29:1165–1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- 112.Sayou C, et al. A SAM oligomerization domain shapes the genomic binding landscape of the LEAFY transcription factor. Nat. Commun. 2016;7:11222. doi: 10.1038/ncomms11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2012;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anders S, Pyl PT, Huber W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics. 2014;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huber, W., von Heydebreck, A., Sültmann, H., Poustka, A. & Vingron, M. Parameter estimation for the calibration and variance stabilization of microarray data. Stat. Appl. Genet. Mol. Biol.2, 1544–6115 (2003). [DOI] [PubMed]
- 117.Tibshirani R. Estimating transformations for regression via additivity and variance stabilization. J. Am. Stat. Assoc. 1988;83:394–405. doi: 10.1080/01621459.1988.10478610. [DOI] [Google Scholar]
- 118.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.MacQueen, J. Some methods for classification and analysis of multivariate observations. In Proc. 5th Berkeley Symposium on Mathematical Statistics and Probability, Vol. 1, 281–297 (Oakland, CA, 1967).
- 120.Massey FJ. The Kolmogorov-Smirnov test for goodness of fit. J. Am. Stat. Assoc. 1951;46:68–78. doi: 10.1080/01621459.1951.10500769. [DOI] [Google Scholar]
- 121.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952;47:583–621. doi: 10.1080/01621459.1952.10483441. [DOI] [Google Scholar]
- 122.Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6:241–252. doi: 10.1080/00401706.1964.10490181. [DOI] [Google Scholar]
- 123.Ackerman-Lavert M, Savaldi-Goldstein S. Growth models from a brassinosteroid perspective. Curr. Opin. Plant Biol. 2019;53:90–97. doi: 10.1016/j.pbi.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 124.Muller, B. & Sheen, J. Arabidopsis Cytokinin signaling pathway. Sci STKE 407, cm5 (2007). [DOI] [PubMed]
- 125.Weijers D, Wagner D. Transcriptional responses to the auxin hormone. Annu. Rev. Plant Biol. 2016;67:539–574. doi: 10.1146/annurev-arplant-043015-112122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptions of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper, its Supplementary information files and public data repositories. Source data are provided with this paper. The ChIP-seq and RNA-seq datasets were deposited to the GEO database (GSE141894). Individual replicates and P values for all figures are provided as a source data file. Source data are provided with this paper.
Scripts for peak to gene annotation can be found at https://github.com/sklasfeld/ChIP_Annotation.