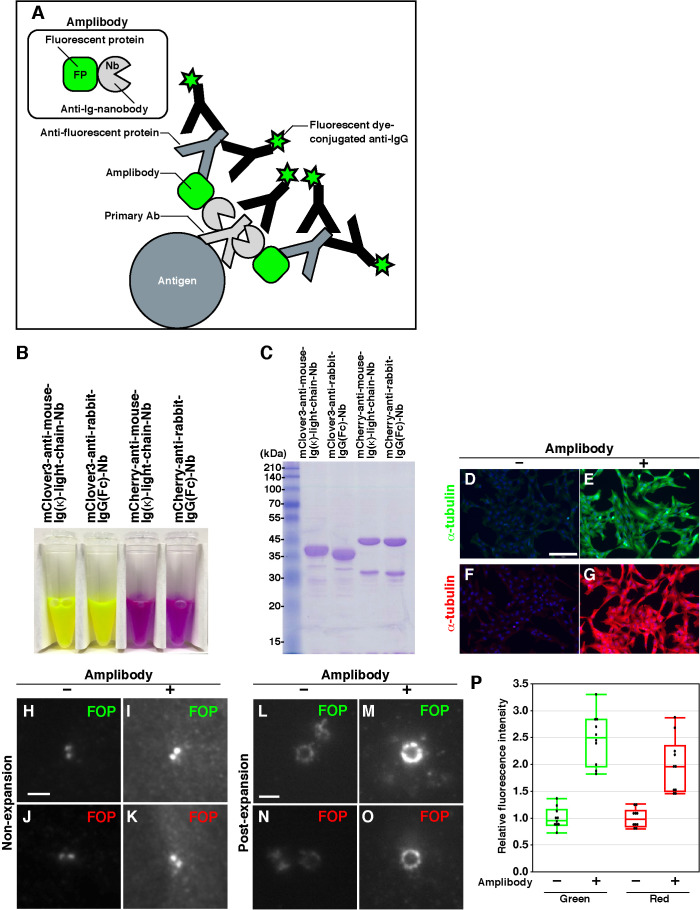
FIGURE 3:
Design of amplibodies and validation of their use in combination with proExM. (A) Schematic diagram of a method for amplifying fluorescence signals using amplibodies, which are anti-Ig Nbs fused to a fluorescent protein. An antigen is recognized by a primary antibody, to which the amplibody binds. Then, an anti–fluorescent protein antibody binds to the fluorescent protein portion of the amplibody, and finally, a fluorescent dye–conjugated secondary antibody binds to the primary and anti–fluorescent protein antibodies. Staining with the fluorescent dye–conjugated antibody can be performed either before or after gelation. (B) Photograph of purified amplibodies. The fluorescence of amplibodies is visible with the naked eye. (C) SDS–PAGE analysis of the purified amplibodies, followed by staining with CBB. (D–O) Validation of the amplification of fluorescence signals with amplibodies by conventional fluorescence microscopy. (D–G) Fixed RPE1 cells were treated with a polyclonal rabbit anti–α-tubulin antibody followed by Alexa488-conjugated (D, E) or Alexa555-conjugated (F, G) anti-rabbit IgG with (E, G) or without (D, F) intervening amplibody treatment (E, mClover3-fused anti-rabbit IgG(Fc) Nb; G, mCherry-fused anti-rabbit IgG(Fc) Nb). (H–O) Nonexpanded (H–K) and expanded (L–O) RPE1 cells were fixed and treated with a monoclonal mouse anti-FOP antibody followed by Alexa488-conjugated (H, I, L, M) or Alexa555-conjugated (J, K, N, O) anti-mouse IgG with (I, K, M, O) or without (H, J, L, N) intervening amplibody treatment (I and M, mClover3-fused anti-mouse Ig κ light chain Nb; K and O, mCherry-fused anti-mouse Ig κ light chain Nb). Scale bars, 150 µm (D–G) and 2 µm (H–O). (P) Comparison of fluorescence signal intensities of FOP-positive rings in expanded cells with or without amplibody treatment (n = 10).