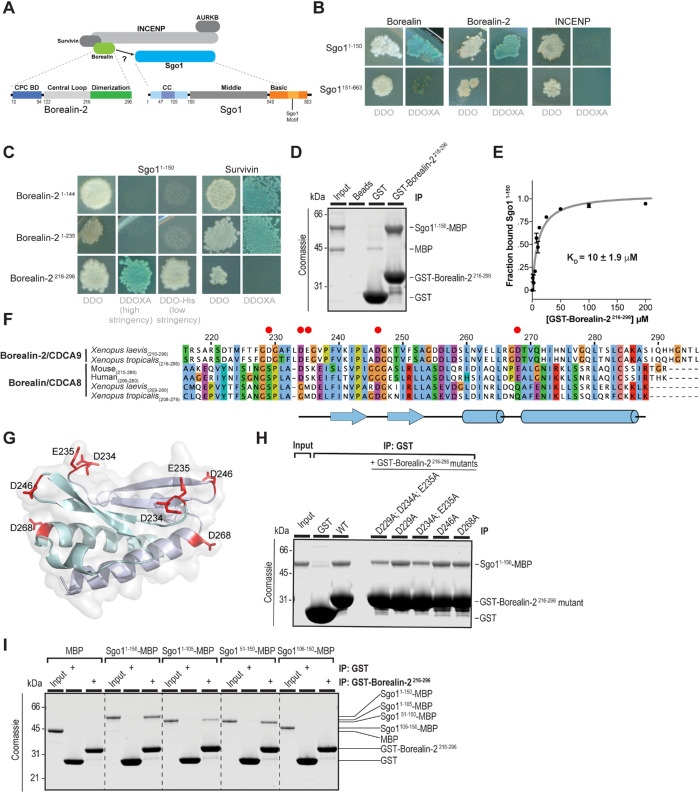
FIGURE 1:
Direct binding of the Borealin C-terminal dimerization domain to Sgo1. (A) Schematic of the chromosomal passenger complex (CPC), which includes Aurora B kinase (AURKB), INCENP, Borealin-2, and Survivin. Aurora B kinase binds to the C-terminal INbox domain of INCENP, and Borealin-2 and Survivin bind to the N-terminal centromere targeting domain of INCENP. Borealin-2 contains three domains: an N-terminal coiled-coil domain through which it interacts with Survivin and INCENP, an unstructured central loop region that binds to nucleosomes, and a C-terminal dimerization domain. Sgo1 contains three domains: an N-terminal coiled-coil domain, a middle domain, and a C-terminal basic domain that includes the Sgo1 motif that interacts with H2AT120ph nucleosomes. (B) Yeast two-hybrid (Y2H) interactions of Sgo1 fragments with Borealin, Borealin-2, and INCENP. White colonies on the left are representative of diploid growth (DDO); blue colonies on the right represent interactions occurring on selective media (DDOXA). Borealin and Borealin-2 interact with Sgo11–150 but not Sgo1151–663. INCENP shows no interaction with Sgo1. Images are representative of four separate experiments. Representative positive and negative Y2H controls used throughout are shown in Supplemental Figure S1B. (C) Y2H interactions of Borealin-2 fragments with Sgo11–150 and Survivin. Borealin-21–144 and Borealin-21–235 interact with Survivin but not Sgo11–150, even under low stringency, and Borealin-2216–296 interacts with Sgo11–150. Images are representative of four separate experiments. See also Supplemental Figure S1, C and D. (D) Binding assay showing GST-Borealin-2216–296 interaction with Sgo11–150 -His6-MBP. Colloidal Coomassie staining of SDS–PAGE gel including input and beads is shown. The input represents 7% total Sgo11–150-His6-MBP incubated with beads. (E) Equilibrium assay to measure affinity of GST-Borealin-2216–296 for Sgo11–150-His6-MBP. Increasing amounts of GST-Borealin-2216–296-bound glutathione sepharose 4B beads were titrated into Sgo11–150-His6-MBP at 2 μM. Beads were pelleted, and samples of the supernatant were separated by SDS–PAGE to determine the fraction bound (see Materials and Methods and Supplemental Figure S1E). Curve is best fit of data to one-site binding equation yielding equilibrium constant of 10 μM. (F) Protein sequence alignment of the dimerization domains from Borealin-2 and Borealin-1 from indicated species. Clustalx coloring was applied to indicate conservation. Numbering denotes amino acid positions from X. laevis Borealin-2. Red circles mark amino acids featured in Figure 1G and mutated in Figure 1H. (G) Homology model of the X. laevis Borealin-2 (CDCA9/Dasra A) dimerization domain based on PDB ID: 2KDD (Bourhis et al., 2009), showing surface-exposed acidic residues. Each monomer is indicated by separate colors. (H) Binding assay showing how mutations in Borealin-2 dimerization domain affect GST-Borealin-2216–296 interaction with Sgo11–150-His6-MBP. Coomassie staining of SDS–PAGE gel including input and beads is shown. The input represents 10% of total Sgo11–150-His6-MBP incubated with beads. (I) Binding assay showing GST-Borealin-2216–296 interaction with Sgo1-His6-MBP truncations. Colloidal Coomassie staining of SDS–PAGE gel including input and beads is shown. The input represents 10% Sgo1-His6-MBP variants incubated with beads.