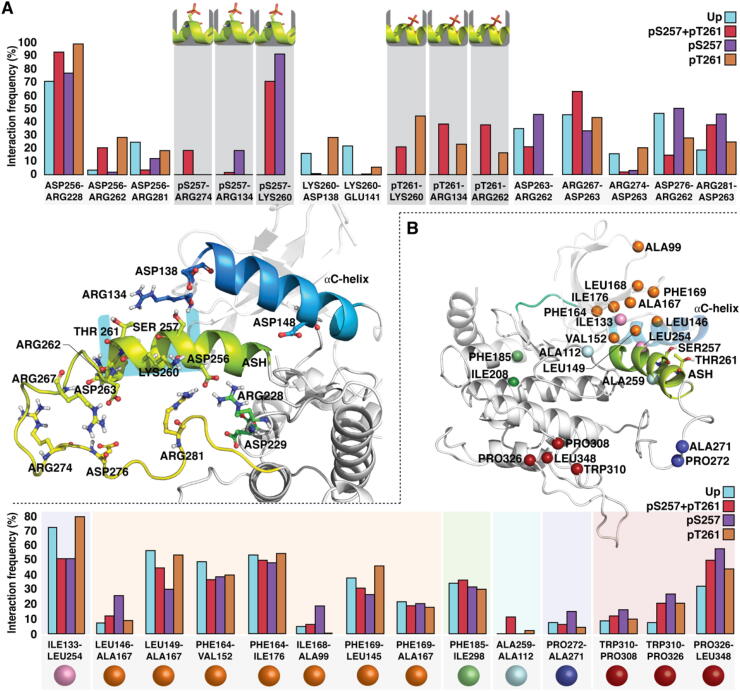
Fig. 4.
Differences in protein interaction networks among different phosphorylation states. (A) Salt-bridges of the activation segment residues ILE250–ALA264 with their interaction frequencies (%). Only the salt-bridges with > 20% differences in their interaction frequencies among systems are shown. (B) Selected hydrophobic interactions of MKK4 and their interaction frequencies among different systems. The locations of the Cα-atoms of the hydrophobic residues are shown in spheres, which are coloured according to different hydrophobic clusters that exhibit linked interactions.