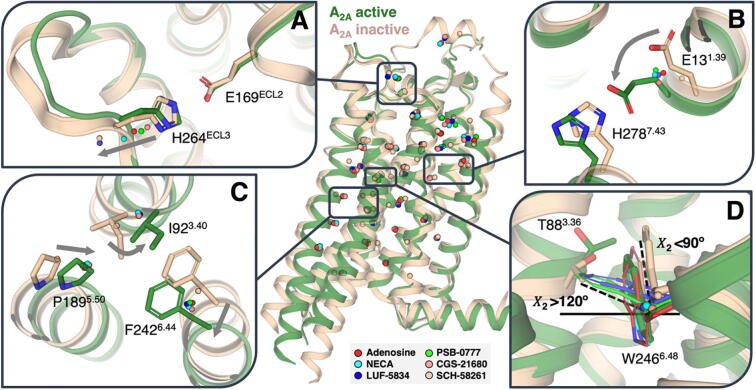
Fig. 5.
Receptor side-chain movements in response to agonists. Plot of the centroids (calculated from 100 snapshots) of the Cβ atoms of a selected group of 34 amino acids (Table S1) located above (in the ligand binding cavity) and below (in the G protein or β-arrestin binding cavity) the “transmission switch” amino acids obtained during 1 μs of MD simulations of A2AR in the presence of the agonists adenosine, NECA, CGS-21680, PSB-0777 and LUF-5834 and a selective antagonist SCH-58261. The distances between the centroids of the agonist-bound conformations and the centroid of the antagonist-bound conformation were statistically correlated with Emax values measured in cAMP production and β-arrestin recruitment (Table S1). (A) Movement of the salt bridge between E169ECL2 and H264ECL3 that is proposed to govern the residence time of ligands. (B) Movement of E131.39, and the nearby H2787.43, that correlates with β-arrestin recruitment. (C) The proposed mechanism of receptor activation at the “transmission switch” amino acids (inward movement of TM 5, an anticlockwise rotation of TM 3, and an outward movement of TM 6, see arrows) is observed but similar for full and partial agonists. (D) Movement of T883.36 and W2466.48 that correlate with cAMP production (Supplementary Fig. S9).