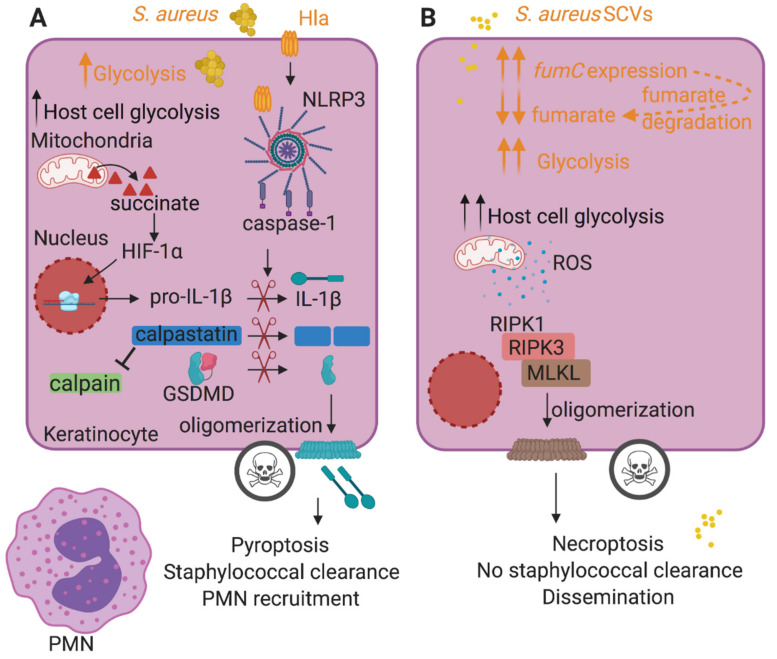
Figure 1.
Host cell death pathways induced by S. aureus influence the outcome of infection. (A) During staphylococcal infection, bacteria and host cells compete for glucose, stimulating bacterial and host cell (e.g., keratinocyte) glycolysis. Host metabolic reprogramming results in succinate production and secretion. Succinate stabilizes the host transcription factor hypoxia-inducible factor-1α (HIF-1α) and increases pro-interleukin-1β (pro-IL-1β) expression. S. aureus toxins such as Hla activate the NLRP3 inflammasome, resulting in caspase-1 activation. Active caspase-1 cleaves pro-IL-1β into the mature form and gasdermin D (GSDMD) into N-terminal and C-terminal fragments. The N-terminal fragment of GSDMD oligomerizes at the cell membrane, forming lytic pores and inducing pyroptotic cell death that eventually recruits polymorphonuclear leukocytes (PMNs) and leads to bacterial clearance. Caspase-1 also cleaves calpastatin, relieving calpain inhibition and promoting pyroptosis. (B) S. aureus induces necroptosis independently of toxin production during keratinocyte infection. S. aureus small colony variants (SCVs), with downregulated toxins, stimulate necroptosis, a caspase-independent cell death modality that does not kill S. aureus and promotes bacterial dissemination, similarly to the WT strain. Their glycolytic nature stimulates keratinocyte glycolysis and promotes the production of reactive oxygen species (ROS) and necroptosis. SCVs sustain glycolysis by increasing fumC expression that promotes the degradation of fumarate, a glycolytic inhibitor. Host-related activities are shown in black font and staphylococcal activities are shown in orange font.