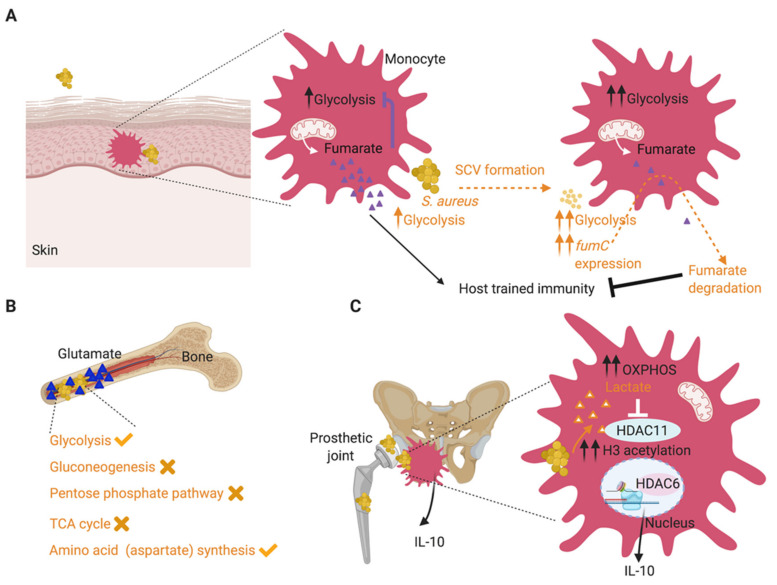
Figure 2.
Adaptive metabolic changes during S. aureus infection promote chronic infection. (A). During skin infection, S. aureus SCVs adapt to local fumarate accumulation by overexpressing fumC to degrade it. This helps sustain glycolysis, given the role of fumarate as a glycolytic inhibitor. Fumarate degradation is also detrimental to the host and abrogates trained immunity, promoting recurrent infections. (B). During staphylococcal bone infection, which is often chronic, S. aureus adapts to the excess glutamate in the infected tissues by stimulating glycolysis and aspartate biosynthesis, both critical pathways for staphylococcal survival in this milieu. Staphylococcal gluconeogenesis, pentose phosphate pathway and TCA cycle were dispensable for survival during osteomyelitis. (C). S. aureus biofilms stimulate a metabolic bias in recruited monocytes, favoring oxidative phosphorylation (OXPHOS) over glycolysis and facilitating their anti-inflammatory activity and biofilm persistence. This metabolic bias is stimulated by S. aureus biofilm-derived lactate, which promotes the production of anti-inflammatory IL-10, by inhibiting histone deacetylase HDAC11 and causing unchecked HDAC6 activity and increased histone acetylation at the Il-10 promoter. Host-related activities are shown in black font and staphylococcal activities are shown in orange font.