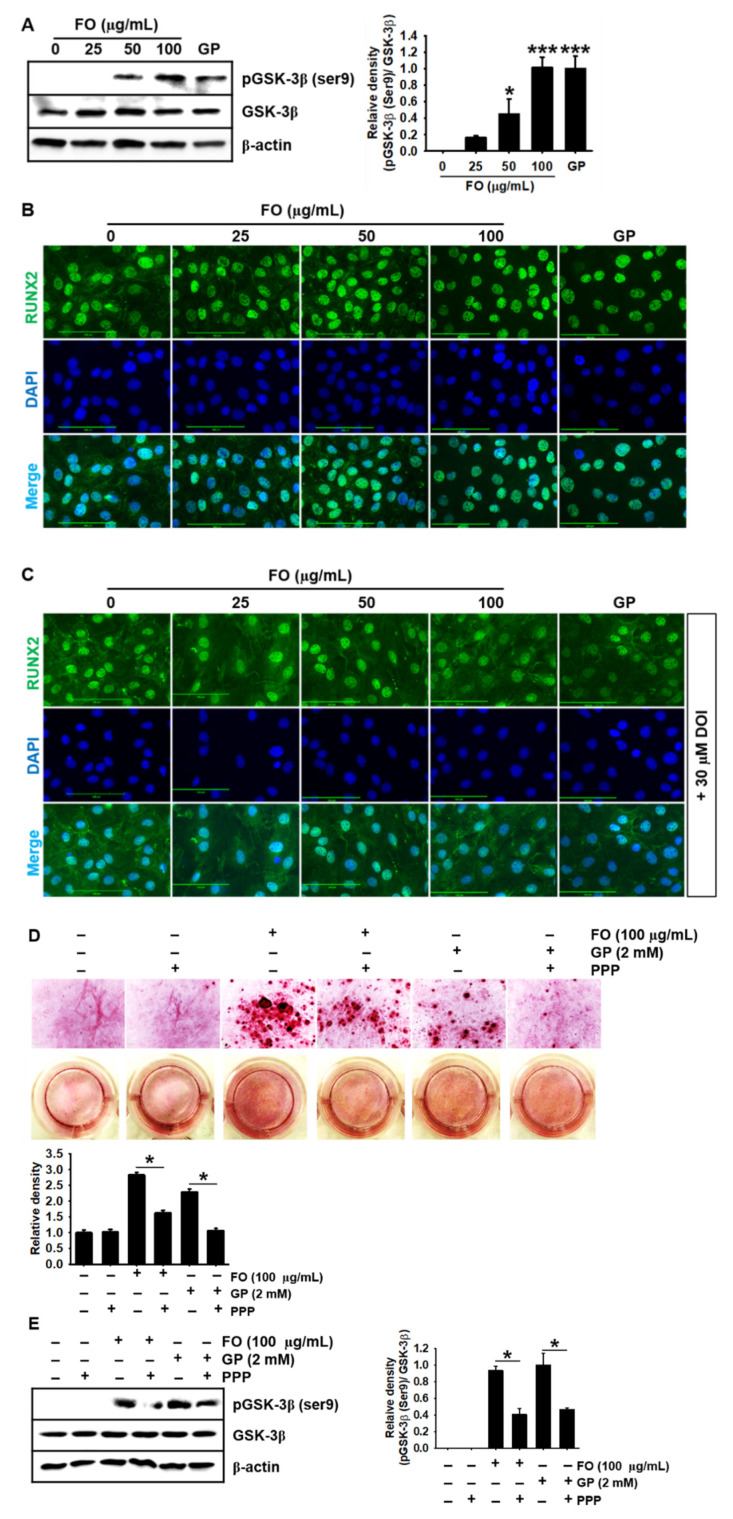
Figure 5.
FO upregulates GSK-3β phosphorylation at Ser9 and thereby promotes the nuclear translocation of RUNX2, leading to calcium deposition. (A) MC3T3-E1 cells were treated with the indicated concentrations of FO (0–100 µg/mL) for 72 h. Total proteins were isolated and Western blotting was performed to measure the protein level of phosphorylated GSK-3β at Ser9. β-glycerophosphate (GP) at 2 mM was used as a positive control. (B and C) MC3T3-E1 cells were seeded on 3% gelatin-coated coverslips and pretreated with 30 µM DOI hydrochloride (DOI) for 2 h prior to treatment with FO or GP. The nuclear translocation of RUNX2 was measured by immunostaining, in the absence (B) and presence (C) of DOI. Scale bar = 100 µm. (D) MC3T3-E1 cells were seeded at a density of 2000 cells/cm2 and pretreated with 1 µM picropodophyllin (PPP) for 2 h prior to stimulation with 100 µg/mL FO and 2 mM GP. After 7 days, the cells were stained with alizarin red to determine the level of calcium deposition. (E) After 3 days in the presence of PPP, total proteins were isolated and Western blotting was performed to measure the level of phosphorylated GSK-3β at Ser9. Total GSK-3β was used as an internal control. The expression of phosphorylated GSK-3β relative to that of total GSK-3β is illustrated in the graphs. Significant differences among the groups were determined using the one-way ANOVA followed by Bonferroni correction. All data are presented as mean ± SEM (* p < 0.05 and *** p < 0.001 vs. untreated MC3T3-E1 cells). FO, fermented oyster (C. gigas) extract.