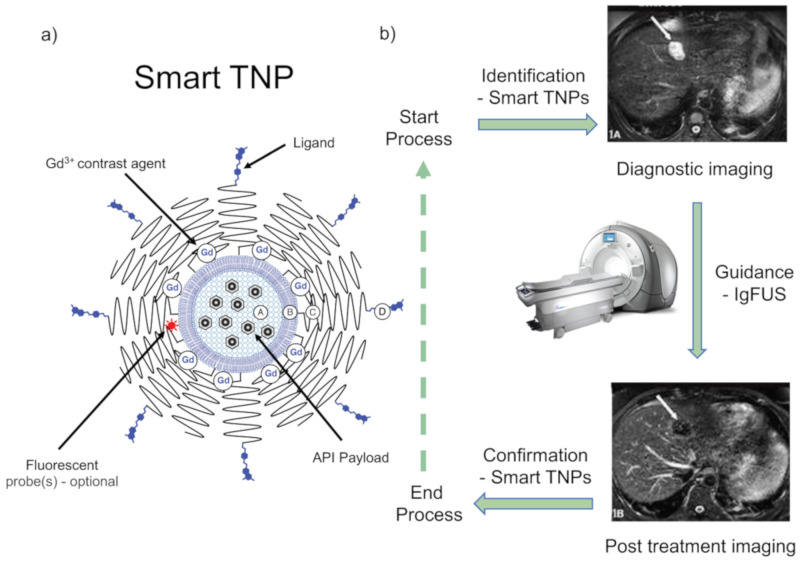
Figure 7.
Schematic representation of a possible PTA for the treatment of CHB in patients. (a) A schematic representation of a theranostic lipid-based nanoparticle (smart TNP) that is enabled for the targeted delivery of an appropriate therapeutic API. According to this paradigm, a smart TNP, as shown, comprises a therapeutic API payload for delivery (A) that is encapsulated within concentric layers of lipids (B) and a layer of stealth-biocompatibility polymer (C) (typically polyethylene glycol), then capped with optional biological cell receptor-specific targeting ligands (D) on its surface. For real-time/diagnostic imaging purposes, a given API should be co-delivered with either a near-infrared fluorophore and/or an MRI contrast agent such as chelated-Gd3+ ions—which act as a positive contrast agent in images of body sections generated by MRI. (b) Any PTA for CHB treatment implies a three-stage process; (1) identification of diseased areas of the liver using a clinically appropriate advanced imaging technique, such as MRI, in conjunction with targeted imaging agents (including imaging lipid nanoparticles [LNPs], or smart TNPs, as above) to show precisely where priority HBV infections of hepatocytes are found; (2) guidance of smart TNPs to high priority HBV infection areas by means of image-guided focused ultrasound (IgFUS) typically aided by MRI; (3) confirmation of effects of targeted API delivery on the zones of CHB infection in the liver by long-term follow up using a clinically appropriate advanced imaging technique, such as MRI. In this instance, smart TNPs will label up disease target areas for as long as infected cells or intracellular infection exists. The schematic anticipates that there may be more than one round of treatment as appropriate.