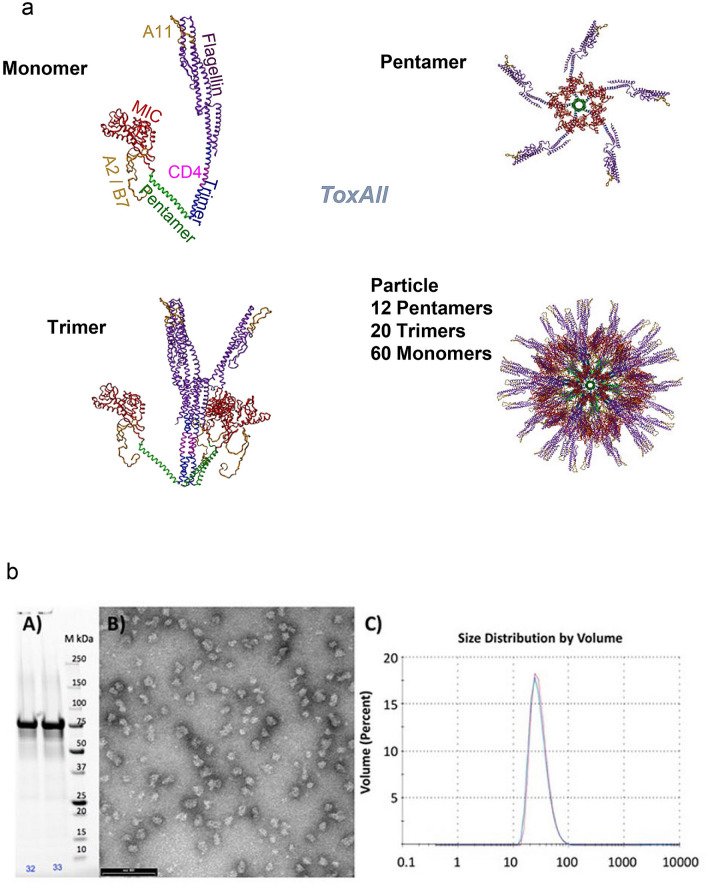
Figure 2.
(a) Computer Model of ToxAll. The core particle is composed of the pentameric and trimeric coiled coils. They are shown in green and blue, respectively. Attached to the trimeric coiled coil is the TLR5 agonist flagellin (purple) while the B cell epitope MIC1 (red) is attached to the pentameric coiled coil. The HLA-A*11:01 CD8+ epitopes (gold) are engineered into flagellin, the CD4+ epitope is engineered into the trimeric coiled coil, the HLA-A*02:01 and HLA-B*07:01 CD8+ epitopes are attached to the N-terminal end of the protein chain. While the B-cell epitope MIC1 appears to be hidden in the assembled particle, the structures of the pentamer and trimer reveal that there is ample void space that allows antibodies to bind to MIC1 in the assembled structure. (b) Biochemical and Biophysical Analysis. Part (A) SDS-PAGE of the purified construct. Part (B) Transmission electron microscopy of ToxAll showing relatively uniform and non-aggregating nanoparticles. The bar corresponds to 100 nm. Part (C) DLS size distribution analysis of ToxAll showing an average nanoparticle size of ~ 40 nm.