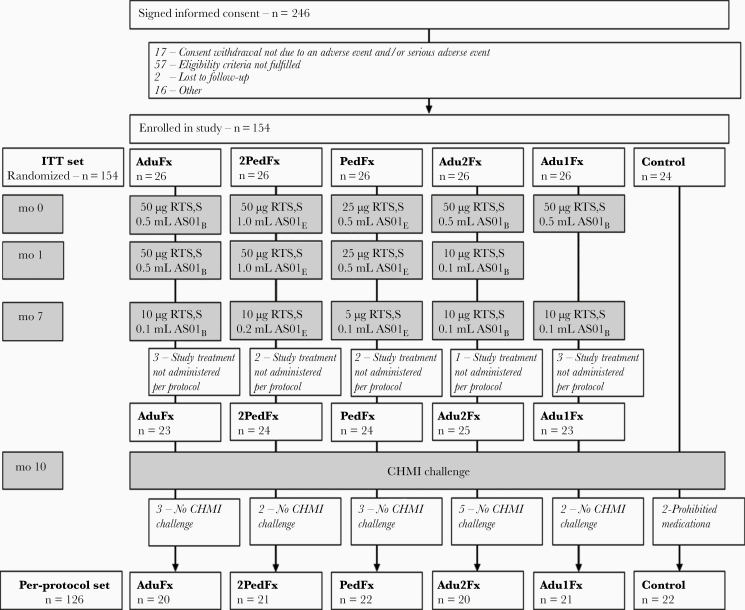
Figure 1.
Consolidated Standards of Reporting Trials flow diagram. Boxes in gray indicate treatment procedures. The intent-to-treat (ITT) set included all participants who received ≥1 dose of a study vaccine and the infectivity controls and was used for analyses of safety. The per-protocol set included all participants in the ITT set who received all vaccinations in accordance with procedures, requirements and limitations specified in the study protocol, who underwent the Plasmodium falciparum challenge, and for whom relevant data were available. AduFx, Adu2Fx, Adu1Fx, PedFx, and 2PedFx indicate study treatment groups. Treatment differences are presented in the individual boxed for months 0, 1, and 7. The protocol for controlled human malaria infection (CHMI) is presented in Methods. Reasons for withdrawal categorized as “Other” include travel, missed vaccination, exclusion criteria met, inability to participate in challenge, pregnancy, and family emergency. Two participants in the infectivity control group were administered presumptive antimalaria medication prohibited in the study protocol (prohibited medication).