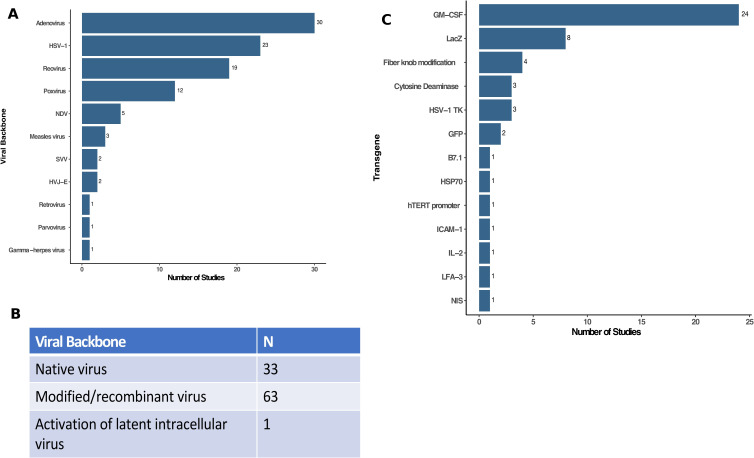
Figure 2.
Characterization of viruses used in oncolytic virus clinical trials. (A) The type (family) of viruses reported in clinical trials were dominated by adenovirus (n=30), HSV-1 (n=23), reovirus (n=19) and poxviruses (n=12) with several other viruses as shown. (B) Table showing the viral backbone used in clinical oncolytic virus studies (eg, native virus, modified viruses, including recombinant or attenuated viruses, or activation of latent intracellular viruses). (C) Transgenes used as payloads in oncolytic viruses. Of the 97 independent clinical trials, 57 used oncolytic viruses without transgenes while 40 had recombinant transgene(s) expressed with GM-CSF (n=24) and LacZ (n=8) being the most common. GM-CSF, granulocyte-macrophage colony-stimulating factor; HSV-1, herpes simplex virus, type 1; HSP70, heat shock protein 70; hTERT, human telomerase reverse transcriptase; HVJ-E, hemagglutinating virus of Japan—Envelope; IL-2, interleukin 2; ICAM-1, intercellular adhesion molecule 1; LFA-3, lymphocyte function associated antigen 3 gene; NDV, Newcastle Disease virus; NIS, sodium iodide symporter; SVV, Seneca Valley virus.